Practice Parameters for the Surgical Treatment of Ulcerative Colitis
Prepared by
The Standards Practice Task Force
The American Society of Colon and Rectal Surgeons
Jeffrey L. Cohen, M.D., Scott A. Strong, M.D., Neil H. Hyman, M.D., W. Donald Buie, M.D., Gary D. Dunn, M.D., Clifford Y. Ko, M.D., Phillip R. Fleshner, M.D., Thomas J. Stahl, M.D., Donald G. Kim, M.D., Amir L. Bastawrous, M.D., W. Brian Perry, M.D., Peter A. Cataldo, M.D., Janice F. Rafferty, M.D., C. Neal Ellis, M.D., Jan Rakinic, M.D., Sharon Gregorcyk, M.D., Paul C. Shellito, M.D., John W. Kilkenny III, M.D., Charles A. Ternent, M.D., Walter Koltun, M.D., Joe J. Tjandra, M.D., Charles P. Orsay, M.D., Mark H. Whiteford, M.D., Jason R. Penzer, M.D.
The American Society of Colon and Rectal Surgeons is dedicated to assuring high-quality patient care by advancing the science, prevention, and management of disorders and diseases of the colon, rectum, and anus. The Standards Committee is composed of Society members who are chosen because they have demonstrated expertise in the specialty of colon and rectal surgery. This Committee was created to lead international efforts in defining quality care for conditions related to the colon, rectum, and anus. This is accompanied by developing Clinical Practice Guidelines based on the best available evidence. These guidelines are inclusive, and not prescriptive. Their purpose is to provide information on which decisions can be made, rather than dictate a specific form of treatment. These guidelines are intended for the use of all practitioners, health care workers, and patients who desire information about the management of the conditions addressed by the topics covered in these guidelines. It should be recognized that these guidelines should not be deemed inclusive of all proper methods of care or exclusive of methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding the propriety of any specific procedure must be made by the physician in light of all of the circumstances presented by the individual patient.
METHODOLOGY
An organized search of Medline, PubMed, and the Cochrane Database of Collected Reviews was performed through September 2004. Key-word combinations included ulcerative colitis, ileal pouch-anal anastomosis, ileostomy, colorectal neoplasm, surgery, ileoproctostomy, and related articles. Directed searches of the embedded references from the primary articles also were accomplished.
INDICATIONS FOR SURGERY
Acute Colitis
1. Patients with clinical evidence of actual or impending perforation should undergo urgent surgery. Level of Evidence: III; Grade of Recommendation: A.
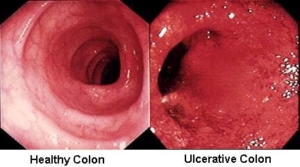
Severe acute colitis affects between 5 to 15 percent of patients with ulcerative colitis. The diagnosis of severe colitis is based on the criteria of Truelove and Witts1 and is defined as colitis with more than six bloody stools per day, fever (temperature, >37.5°C), tachycardia (heart rate, >90 beats per minute), anemia (hemoglobin, <75 percent of normal), and elevated sedimentation rate (ESR, >30 mm per hour).2 Alternatively, toxic, or fulminant, colitis is characterized by more than ten bloody stools per day, fever (temperature, >37.5°C), tachycardia (heart rate, >90 beats per minute), anemia (transfusion required), elevated sedimentation rate (ESR, >30 mm per hour), colonic dilation on radiography, and abdominal distention with tenderness.2 When the colonic distention of the transverse colon exceeds 6 cm, the diagnosis becomes toxic megacolon.3,4 Surgery is required in 20 to 30 percent of patients with toxic colitis.5
Perforation in patients with toxic colitis has a high mortality rate, which ranges from 27 to 57 percent regardless of whether the perforation is contained or free.6,7 The mortality rate increases as the time interval between perforation and surgery increases.6,8 Patients with toxic colitis receiving surgical intervention before perforation have a significantly better outcome than those operated on after perforation.7,8 However, there are few “hard” signs of impending perforation in patients with toxic colitis. Perforation can occur without dilation and these patients often do not exhibit classic signs of peritonitis.6 Persistent or increasing colonic dilation, pneumatosis coli, worsening local peritonitis, and the development of multiple organ failure can be signs of impending or actual perforation. 7,9,10 Localized peritonitis may reflect only local inflammation or may be a sign of impending perforation. 11
The development of multisystem organ failure (MSOF) is an ominous sign. In a series of 180 patients with toxic colitis, 11 developed MSOF. The overall mortality in the entire group was 6.7 percent; however, of the 12 patient deaths, 8 occurred in patients with MSOF.12
2. Patients whose condition worsens on medical therapy or who fail to make significant improvement after a period of 48 to 96 hours of appropriate medical therapy should be considered for surgery. Level of Evidence: III; Grade of Recommendation: B.
Patients are judged to have failed medical therapy if their condition worsens while on medical therapy or their condition fails to improve after a period of initial stabilization. Limited evidence suggests that intravenous cyclosporine is more effective than standard steroid- based treatment for severe colitis13–15 and has been advocated as a second-line agent before colectomy. The need for and timing of surgery in patients whose condition seems to “plateau” after a period of initial improvement often is difficult to judge. However, patients with more than eight stools per day or three to eight stools and a C-reactive protein > 45 mg/ml after three days of therapy have an 85 percent chance of requiring colectomy during the same hospitalization, regardless of whether corticosteroid or cyclosporine treatment is used.16 Furthermore, persistent colonic distention seems to characterize a subgroup of patients who respond poorly to medical therapy and are at increased risk for the development of megacolon.17 Prolonged observation of these patients may risk exhaustion of their physiologic reserve but does not necessarily increase perioperative morbidity. 18 Most series define a period of 48 to 96 hours after which surgery is indicated if the patient fails to improve,5,8,9 although evidence specifying the most appropriate time period for a trial of medical therapy, especially with “second-line” agents, is lacking.
Intractability
1. Surgery is indicated in ulcerative colitis when medical therapy is ineffective. Level of Evidence: III; Grade of Recommendation: B.
Intractability is one of the most common surgical indications for ulcerative colitis. Medical therapy can fail for several reasons. Symptoms may be insufficiently controlled despite an intensive medical regimen and the patient is unable to achieve an acceptable quality of life. Alternatively, the response to treatment may be adequate, but the risks of chronic medical therapy (especially long-term corticosteroids) may be excessive. Patients also may be unable to tolerate the deleterious side effects of medical therapy. Patients who are noncompliant with treatment regimens might be candidates for surgical management. The postoperative quality of life for patients with ulcerative colitis is improved after colectomy.19–23
Growth failure in children is another form of intractability that may require colectomy. Surgery should be considered if growth failure persists despite maximal nutritional and medical therapy.24 Substantial disability from colectomy-responsive extraintestinal manifestations also may prompt resection.
Cancer Risk
1. Patients with long-standing ulcerative colitis should undergo endoscopic surveillance. Level of Evidence: IV; Grade of Recommendation: B.
Although it is clear that patients with longstanding ulcerative colitis have an increased risk of colorectal cancer, its magnitude has been difficult to estimate. A recent meta-analysis estimated the risk of colorectal cancer for a patient with colitis to be 2 percent at 10 years, 8 percent at 20 years, and 18 percent after 30 years of disease.25 Surveillance colonoscopy has been recommended in these patients despite a lack of clear evidence that shows surveillance prolongs survival in patients with ulcerative colitis. Carcinomas tend to be detected at an earlier stage in persons who are undergoing surveillance colonoscopy, and these patients have a better prognosis.26,27
Patients with extensive colitis (microscopic disease proximal to the splenic flexure) should be advised to undergo a screening endoscopy after eight years of disease symptoms and should have a surveillance colonoscopy performed every one to two years. If a person suffers from left-sided disease (i.e., microscopic disease distal to the splenic flexure yet proximal to the rectum), he or she may begin the same surveillance program after 15 years of disease symptoms despite a lack of direct supporting evidence for this duration-dependent stratification.28–30 Surveillance colonoscopies should be ideally performed when the disease is in remission to minimize confusion regarding neoplasia. Because it is necessary to take at least 33 biopsies from the colon and rectum to achieve 90 percent sensitivity,31 it is reasonable to obtain four quadrant random biopsies at 10-cm intervals along the large intestine, taking particular care to biopsy any strictures or mass-like lesions while avoiding any nonsuspicious pseudopolyps. Polyps that appear potentially dysplastic can be removed by polypectomy, and the adjacent flat mucosa also should be biopsied to exclude dysplasia. Recent enthusiasm has emerged for targeted biopsies with chromoendoscopy by using pancolonic indigo carmine dye spraying. 32,33
Several studies indicate patients with concomitant primary sclerosing cholangitis (PSC) are at a higher risk of colorectal neoplasia.34 The absolute cumulative risk of cancer or dysplasia in this subset of patients has been estimated to be 9 percent after 10 years, 31 percent after 20 years, and 50 percent after 25 years of colitis.34 Patients with PSC often have quiescent colitis, so it is difficult estimating the precise onset of disease in this subgroup. For the above reasons, it is recommended that such patients should undergo annual surveillance colonoscopy.
2. Total proctocolectomy is recommended for patients with carcinoma, nonadenoma-like dysplasia associated lesion or mass (DALM), high-grade dysplasia, and low-grade dysplasia in a stricture that is symptomatic or impassable during colonoscopy. The diagnosis of dysplasia should ideally be confirmed by two independent expert gastrointestinal histopathologists. Level of Evidence: Class III; Grade of Recommendation: C.
Dysplasia detection by conventional histopathologic assessment of colonoscopic biopsies remains the “gold standard” to identify patients at highest risk of developing cancer in ulcerative colitis.35 Ten prospective surveillance programs published before 1994 demonstrated that in patients diagnosed with a DALM, 43 percent had a synchronous cancer at immediate colectomy.36 The risk of cancer at immediate colectomy was 42 percent for high-grade dysplasia and 19 percent for low-grade dysplasia. The risk of developing high-grade dysplasia, DALM, or cancer was 2.4 percent in patients without dysplasia on initial screening, 18 percent for those with “indefinite dysplasia,” and 29 percent for those with low-grade dysplasia. In another review, 9 of 18 patients identified with ulcerative colitis and low-grade dysplasia developed advanced neoplastic lesions, which were defined as adenocarcinoma, raised dysplasia, or highgrade dysplasia, during follow-up.37 Moreover, a surveillance study indicated the five-year predictive value for cancer or high-grade dysplasia in patients with low-grade dysplasia was 54 percent.38
However, in a conflicting study, 60 patients with low-grade dysplasia in flat mucosa found during endoscopy were followed for an average of ten years; low-grade dysplasia was found at several locations and during repeated colonoscopies in 73 percent of cases, but progression to high-grade dysplasia or a dysplasia-associated lesion/mass occurred in only 11 patients (18 percent).39 The high rates of interobserver variation between histopathologists further confounds the management of low-grade dysplasia. 40–43
There also is controversy regarding the natural history of adenoma-like DALMs. Specifically, in the absence of dysplasia in neighboring flat mucosa, recent reports suggest that adenoma-like DALMs can be effectively removed by colonoscopic resection without placing the patient at increased risk of developing future dysplasia or carcinoma.44–46
Patients should be encouraged to take prescribed 5-aminosalicylate (ASA) medication, because recent literature suggests that regular consumption of 5-ASA compounds may reduce their colorectal cancer risk.47–49 In a case control study,49 regular 5-ASA therapy reduced cancer risk by 75 percent (odds ratio (OR), 0.25; 95 percent confidence interval (CI), 0.13– 0.48; P < 0.00001). Another study demonstrated that pharmacologic therapy, especially sulfasalazine, was associated with a significant protective effect (relative risk (RR), 0.38; 95 percent CI, 0.2–0.69) independent of disease activity.47 The risk of developing cancer was 5 of 152 (3 percent) in a group who took longterm 5-ASA and 5 of 16 (31 percent) in those who had had their treatment stopped or did not comply with therapy.48
3. Patients with ulcerative colitis who develop a stricture, especially with long-standing disease, should undergo resection. Level of Evidence: III, Grade of Recommendation: A.
Strictures develop in 5 to 10 percent of patients with ulcerative colitis. Although the majority of strictures are benign, as many as 25 percent will be malignant, and malignant strictures account for up to 30 percent of cancers occurring in ulcerative colitis patients. Strictures that arise on a background of long-standing disease, originate proximal to the splenic flexure, or cause obstructive symptoms are more likely to be malignant. 50 Endoscopic biopsy of strictures can reveal dysplasia or malignancy51 but may be unreliable because of sampling error and the more infiltrating nature of colitis-associated malignancies.50,52
SURGICAL OPTIONS
Emergency
1. The most appropriate operative procedure for emergency surgery in ulcerative colitis is total or subtotal abdominal colectomy with end ileostomy. Level of Evidence: III, Grade of Recommendation: B.
The surgical alternatives in the acute setting are designed to restore patient health with the greatest reliability and minimal risk while preserving reconstructive options after the patient has recovered. Subtotal colectomy with end ileostomy and Hartmann’s closure of the distal bowel or creation of a mucous fistula is a safe and effective approach.18,53 This procedure removes the majority of the inflamed bowel with a comparatively straightforward operation and avoids pelvic dissection as well as an intestinal anastomosis. 54,55 Compared with intraperitoneal closure of the rectal stump, extrafascial placement of a closed rectosigmoid stump may be associated with fewer pelvic septic complications and facilitates subsequent pelvic dissection.56 Transanal drainage of the distal stump may further decrease the risk of pelvic sepsis.57
The resected colon specimen should be histopathologically examined for confirmation of ulcerative colitis or Crohn’s disease because the likelihood of an altered diagnosis is appreciable after colectomy.18,53 In patients with ulcerative colitis, a completion proctectomy and ileal pouch-anal anastomosis (IPAA) often can be safely performed at a later date to remove the remaining disease and restore intestinal continuity. If the diagnosis is Crohn’s disease and the rectum is reasonably compliant and distensible, consideration may be given to an ileorectal anastomosis.
Elective Surgery
1. Total proctocolectomy with ileostomy is an appropriate surgical alternative for patients with ulcerative colitis. Level of Evidence: III; Grade of Recommendation: B.
Proctocolectomy with ileostomy has been the conventional operative approach for patients with ulcerative colitis and may be considered a benchmark procedure to which all other operations are compared. 58,59 It has been established as a safe, curative operation that permits most patients to live a full, active lifestyle.20,60 Although restorative proctocolectomy with IPAA has become increasingly popular during the past two decades, proctocolectomy with ileostomy can still be considered the first-line procedure for patients who choose not to undergo a restorative proctocolectomy and for those at significant risk for pouch failure, such as patients with impaired anal sphincter muscles, previous anoperineal disease, or limited physiologic reserve secondary to comorbid conditions.61
The operation, however, does have recognized complications. Although stoma-associated problems, such as prolapse, are probably most frequent,62 other complications that are common to any abdominal/ pelvic procedure also have been recognized.62,63 These include small-bowel obstruction, infection/ fistula, persistent pain, unhealed perineal wound, sexual and bladder dysfunction, and infertility.64 In one recent study of 44 patients, the long-term complication rate of proctocolectomy with permanent ileostomy was significantly lower than restorative proctocolectomy (26 vs. 52 percent).63
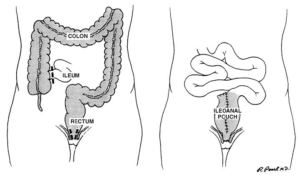
2. Total proctocolectomy with ileal pouch-anal anastomosis is an appropriate operation for most patients with ulcerative colitis. Level of Evidence: III; Grade of Recommendation: A.
Total proctocolectomy with IPAA has become the most commonly performed procedure for patients with ulcerative colitis requiring elective surgery. The operation is relatively safe and durable,65,66 associated with an acceptable morbidity rate (19 to 27 percent), 67,68 an extremely low mortality rate (0.2–0.4 percent),67,68 and a quality of life that approaches that of the normal population.69–72 The complications of the procedure include those of any major abdominal operation: risks arising from the pelvic dissection, such as infertility or sexual dysfunction, and pouchspecific complications, such as pouchitis.73–81
a. Total proctocolectomy with IPAA may be appropriately offered to selected ulcerative colitis patients with concomitant colorectal cancer. Level of Evidence: IV; Grade of Recommendation: C.
Studies examining the use of IPAA in patients with invasive cancers of the colon or upper rectum without distant metastases have yielded somewhat conflicting findings. In several series, ulcerative colitis patients with a concomitant carcinoma had a rate of postoperative complications and functional results comparable to colitis patients without cancer; metastatic disease developed in a small number of patients.82–85 In contrast, a separate study revealed that nearly 20 percent of ulcerative colitis patients with cancer who underwent an IPAA subsequently died of metastatic disease. 86 A more conservative management approach has been advocated by some who recommend an abdominal colectomy with ileostomy followed by a restorative proctectomy after an observation period of at least 12 months to better assure that no recurrent disease develops.87
Metastatic disease is generally considered a contraindication to IPAA. These patients should usually be managed with segmental colectomy or abdominal colectomy with anastomosis to facilitate early discharge and allow them to spend the rest of their lives relatively free of complications. Another group of patients who may not be eligible for IPAA are those with invasive carcinomas of the mid or low rectum, because basic principles of cancer surgery may be compromised. Adjuvant radiotherapy, if indicated, should be performed preoperatively whenever possible, because postoperative radiotherapy is associated with a high incidence of pouch loss secondary to radiation enteritis and poor pouch function.83 Ulcerative colitis patients with cecal cancers represent another unique subgroup of patients. If a long segment of adjacent distal ileum with its mesenteric vessels must be sacrificed, difficulties with positioning of the reservoir into the pelvis may ensue, and an ileostomy may be necessary if a tension-free anastomosis cannot be attained.
b. Total proctocolectomy with IPAA may be appropriately offered to selected elderly patients with ul- Vol. 48, No. 11 PRACTICE PARAMETERS FOR ULCERATIVE COLITIS 2001 cerative colitis. Level of Evidence: III; Grade of Recommendation: C.
Many groups have demonstrated that IPAA in the elderly patient is safe and feasible.88–91 Chronologic age should not itself be used as an exclusion criterion. However, careful consideration should be given to other comorbidities, as well as the patient’s mental status and anal sphincter function. Pouch procedures are feasible in suitably motivated elderly individuals who understand the risks and potential function difficulties that often accompany this procedure. Although some series have found that bowel frequency remains constant in the first decade after the surgical procedure,92 others have found the number of daytime and nighttime stools increases as does the likelihood of fecal incontinence.65,93
c. Mucosectomy and double-stapled procedures are both appropriate techniques in most circumstances. Level of Evidence: II; Grade of Recommendation: A.
The potential advantages of the double-stapled approach include enhanced technical ease because it avoids mucosectomy and the perineal phase of the operation, there is less tension on the anastomotic suture line, and possibly improved functional results. Sphincter injury is minimized and the anal transition zone with its abundant supply of sensory nerve endings is preserved. Conversely, short segment inflammation94,95 and perianastomotic zone carcinoma96–98 are legitimate concerns, highlighting the importance of performing the anastomosis to the top of the anal canal. Three prospective, randomized trials have demonstrated no significant difference in perioperative complications or functional results for patients in whom a mucosectomy was performed vs. those patients in whom the proximal anal canal mucosa was preserved.99–101 It is important that the surgeon performing an IPAA be familiar with both techniques in the event of failure or inability to use a surgical stapler or when a handsewn anastomosis is contemplated but anastomotic tension is excessive. Patients should be followed in a surveillance program with biopsies of the retained columnar mucosa performed at least every two years beginning eight to ten years after the onset of their initial disease symptoms.102
d. Pouch configuration may be chosen based on individual preference. Level of Evidence: II; Grade of Recommendation: B.
Although the initial ileal reservoir created by Parks in the late 1970s was a triple-loop S-pouch,103 other pouch configurations have been described in an attempt to reduce pouch complications and improve functional outcome. These include the double-loop J-pouch, the lateral isoperistaltic H-pouch, and the quadruple-loop W-pouch.104–106 S-pouches were initially plagued with evacuation problems associated with a long (5 cm) exit conduit, frequently requiring pouch catheterization.103 With shortening of the exit conduit to 2 cm, mandatory catheterization has been substantially reduced.107 The long outlet tract formed in the H-pouch also was associated with pouch distention, stasis, and pouchitis.108 The Wpouch has been advocated because of a greater capacity. 106 However, two randomized trials comparing the J-pouch and W-pouch did not substantiate an improvement in functional outcomes.109,110 In one study,109 the median number of stools per day was the same in patients with a J-pouch or W-pouch, and there was no difference between the two reservoirs in the rates of incontinence, urgency, soiling, and the use of antidiarrheal agents. Another controlled study110 also demonstrated similar functional results between J-pouch and W-pouch one year after surgery. An S-pouch can provide additional length (2–4 cm) compared with the J-pouch and may help minimize anastomotic tension.111 However, the 2-cm exit conduit of the S-pouch may elongate with time, and obstructive defecation can develop.
e. A diverting loop ileostomy may be reasonably omitted in some patients. Level of Evidence: III; Grade of Recommendation: B.
Retrospective and prospective trials suggest that one-stage restorative proctocolectomy can be safely performed in selected patients by experienced surgeons. The one-stage procedure is associated with a more challenging early recovery,112 as well as a slightly increased rate of anastomotic disruption and pelvic sepsis.113–121 Although some disagree,122 these complications should usually be managed with fecal diversion118,119 because of concerns about compromised functional outcome and resultant pouch loss.123 Despite aggressive nonoperative and operative measures, the estimated cumulative three-, fiveand ten-year rate of pouch failure in all patients with septic complications is 20, 31, and 39 percent, respectively. 121 This highlights the need for great caution when considering pelvic pouch surgery without temporary diversion. Single-stage IPAA avoids the risks of ileostomy closure, which include anastomotic leaks from the closure site and an increased incidence of postoperative small-bowel obstruction that often mandates hospitalization or laparotomy.119,124–127 In 2002 COHEN ET AL Dis Colon Rectum, November 2005 general, selective omission of the ileostomy may be considered safe when the anastomosis appears intact, is under no tension, the procedure is not complicated by excessive bleeding or other technical difficulties, and the patient is not on high doses of corticosteroids before surgery.84,116,117,121–123,127,128
f. Routine surveillance of ileal pouches for dysplasia in the ileal mucosa is not warranted. Level of Evidence: III; Grade of Recommendation: B. A decrease in villous height and increase in concentration of crypts have been observed in most ileal pouches.129 These metaplastic changes of the ileal mucosa to a colonic type mucosa are considered adaptations to the reservoir function of the pouch. This transformation also may be driven by the chronic inflammation frequently observed in these pouches.130 Inflammatory changes could theoretically lead to dysplasia and cancer in the ileal mucosa. However, dysplastic and neoplastic transformation within the pouch seems to be extremely rare.131–133
g. Pouchitis is common after IPAA and readily managed with antibiotics in most circumstances. Level of Evidence: II; Grade of Recommendation: A.
The most frequent long-term complication after IPAA for ulcerative colitis is a nonspecific inflammation of the ileal pouch known as pouchitis.67,68,92,134 The presence of extraintestinal manifestations of ulcerative colitis before colectomy, especially primary sclerosing cholangitis, has been associated with an increased incidence of pouchitis.134,135 It is unclear whether the presence of backwash ileitis or extent of disease predict the likelihood of ultimately developing pouchitis.136–138 The etiology of this nonspecific inflammation is unclear but may be the result of an overgrowth of anaerobic bacteria.139,140 Presenting symptoms usually include abdominal cramps, fever, pelvic pain, and an increase in stool frequency. Clinical diagnosis may require confirmation by endoscopy and pouch mucosal biopsy, because clinical symptoms alone can be misleading.141 However, it seems that histologic evaluation may be omitted without compromising diagnostic accuracy.142 Treatment of pouchitis relies primarily on antibiotics, such as metronidazole and ciprofloxacin.143–145 Probiotics have been used successfully in pouch patients to provide prophylaxis against pouchitis and to maintain remission in chronic pouchitis.146,147 In antibiotic refractory cases, budesonide enemas or other medical treatments may be useful.148 Patients suffering with chronic pouchitis should be assessed for a diagnosis of Crohn’s disease. Uncommonly, an ileostomy with or without pouch excision is required for severe refractory pouchitis.145
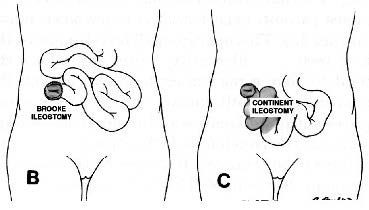
3. Continent ileostomy is an alternative surgical option for patients with ulcerative colitis who are not eligible for or have had a failed restorative proctocolectomy. Level of Evidence: III; Grade of Recommendation: B.
The present role of the continent ileostomy is primarily confined to patients with poor sphincter function, a failed IPAA, or those who are dissatisfied with a conventional Brooke ileostomy.149,150 This reduced role is the result of the success of the IPAA and the high rate of early and late complications associated with the continent ileostomy.151
Early complications are seen in approximately onequarter of patients, most commonly sepsis (secondary to suture line leaks, fistulas, and stomal necrosis) and obstruction.152,153 Late complications occur in up to 50 percent of patients and include incontinence and obstruction secondary to disruption or dysfunction of the valve; valve revision is required in up to 60 percent of patients.151 Although valve prolapse has been reduced with stapling techniques,150,154 the overall pouch failure rate has not decreased.155
The cumulative success rate of the continent ileostomy in a recent study was 71 percent at 29 years.151 The failure rate is greater after secondary construction after a failed IPAA (46 percent) than after primary construction (23 percent).155 For the two-thirds of patients with a functional continent ileostomy, the reported quality of life is similar to that described for patients with IPAA.151,155,156
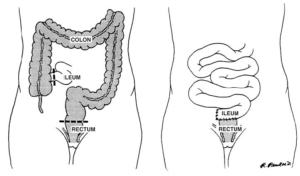
4. Total abdominal colectomy with ileoproctostomy is an acceptable surgical approach in a highly selected group of patients with ulcerative colitis. Level of Evidence: III; Grade of Recommendation: B.
Because the performance of a total abdominal colectomy with ileoproctostomy requires a relatively normal rectum to create a safe anastomosis, severe rectal inflammation or a marked decrease in rectal distensibility are contraindications to the procedure. 157,158 Severe anoperineal disease, although unusual in ulcerative colitis, also precludes an ileorectal anastomosis.159 Other contraindications to this operation are colonic dysplasia or carcinoma in a potentially curative situation.160
Whereas the benefits of total abdominal colectomy with ileoproctostomy are its relative simplicity and predictability compared with IPAA, the disadvantages are related to the long-term durability of the procedure. Studies demonstrate a 12 to 50 percent failure Vol. 48, No. 11 PRACTICE PARAMETERS FOR ULCERATIVE COLITIS 2003 rate with follow-up of more than six years.161–163 In addition, the theoretical risk of developing cancer in the remaining rectum should be considered when counseling the patient and other options discussed. Although the incidence of developing cancer seems to be low (0–6 percent with long-term followup), 155,163–165 patients undergoing total abdominal colectomy with ileorectal anastomosis must be willing to undergo annual endoscopic screening.158–163
REFERENCES
1. Truelove SC, Witts LF. Cortisone in ulcerative colitis: final report on a therapeutic trial. BMJ 1955;2:1041–8. 2. Hanauer SB. Drug therapy: inflammatory bowel disease. N Engl J Med 1996;334:841–8. 3. Jones JH, Chapman M. Definition of megacolon in colitis. Gut 1969;10:562–4. 4. Katz JA. Medical and surgical management of severe colitis. Semin Gastrointest Dis 2000;11:18–32. 5. Present DH. Toxic megacolon. Med Clin North Am 1993;77:1129–48. 6. Greenstein AJ, Barth JA, Sachar DB, Aufses AH Jr. Free colonic perforation without dilatation in ulcerative colitis. Am J Surg 1986;152:272–5. 7. Heppell J, Farouk E, Dube S, Peloquin A, Morgan S, Bernard D. Toxic megacolon. An analysis of 70 cases. Dis Colon Rectum 1986;29:789–92. 8. Greenstein AJ, Sachar DB, Gibas A, et al. Outcome of toxic dilatation in ulcerative and Crohn’s colitis. J Clin Gastroenterol 1985;7:137–43. 9. Berg DF, Bahadusingh AM, Kaminski DL, Longo WE. Acute surgical emergencies in inflammatory bowel disease. Am J Surg 2002;184:45–51. 10. St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg 2003;138:68–75. 11. Roy M. Inflammatory bowel disease. Surg Clin North Am 1997;77:1419–36. 12. Caprilli R, Latella G, Vernia P, Frieri G. Multiple organ dysfunction in ulcerative colitis. Am J Gastroenterol 2000;95:1258–62. 13. Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994;330:1841–5. 14. D’Haens G, Lemmens L, Geboes K, et al. Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology 2001;120:1323–9. 15. Shibolet O, Regushevskaya E, Brezis M, Soares-Weiser K. Cyclosporine A for induction of remission in severe ulcerative colitis. Cochrane Database of Systematic Reviews. 1, 2005. 16. Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut 1996;38:905–10. 17. Latella G, Viscido A, Frieri G, et al. GI distension in severe ulcerative colitis. Am J Gastroenterol 2002;97: 1169–75. 18. Hyman NH, Cataldo P, Osler T. Urgent subtotal colectomy for severe inflammatory bowel disease. Dis Colon Rectum 2005;48:70–3. 19. McLeod RS, Churchill DN, Lock AM, Vanderburgh S, Cohen Z. Quality of life of patients with ulcerative colitis preoperatively and postoperatively. Gastroenterology 1991;101:1307–13. 20. McLeod RS, Baxter NN. Quality of life of patients with inflammatory bowel disease after surgery. World J Surg 1998;22:375–81. 21. Sagar PM, Lewis W, Holdsworth PJ, Johnston D, Mitchell C, MacFie J. Quality of life after restorative proctocolectomy with a pelvic ileal reservoir compares favorably with that of patients with medically treated colitis. Dis Colon Rectum 1993;36:584–92. 22. Thirlby RC, Land JC, Fenster LF, Lonborg R. Effect of surgery on health-related quality of life in patients with inflammatory bowel disease: a prospective study. Arch Surg 1998;133:826–32. 23. Muir AJ, Edwards LJ, Sanders LL, et al. A prospective evaluation of health-related quality of life after ileal pouch anal anastomosis for ulcerative colitis. Am J Gastroenterol 2001;96:1480–5. 24. Berger M, Gribetz D, Korelitz BI. Growth retardation in children with ulcerative colitis: the effect of medical and surgical therapy. Pediatrics 1975;55:459–67. 25. Eaden JA, Abrams K, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35. 26. Hata K, Watanabe T, Kazama S, et al. Earlier surveillance colonoscopy programme improves survival in patients with ulcerative colitis associated colorectal cancer: results of a 23-year surveillance programme in the Japanese population. Br J Cancer 2003;89:1232–6. 27. Mpofu C, Watson AJ, Rhodes JM. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database of Systematic Reviews. 1, 2005. 28. Riddell RH. Screening strategies in gastrointestinal cancer. Scand J Gastroenterol Suppl 1990;175:177–84. 29. Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale— update based on new evidence. Gastroenterology 2003;124:544–60. 30. Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2004;99: 1371–85. 31. Rubin CE, Haggitt RC, Burmer GC, et al. DNA aneuploidy in colonic biopsies predicts future development 2004 COHEN ET AL Dis Colon Rectum, November 2005 of dysplasia in ulcerative colitis. Gastroenterology 1992;103:1611–20. 32. Rutter MD, Saunders BP, Schofield G, Forbes A, Price AB, Talbot IC. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut 2004;53:256–60. 33. Sada M, Igarashi M, Yoshizawa S, et al. Dye spraying and magnifying endoscopy for dysplasia and cancer surveillance in ulcerative colitis. Dis Colon Rectum 2004;47:1816–23. 34. Jayaram H, Satsangi J, Chapman RW. Increased colorectal neoplasia in chronic ulcerative colitis complicated by primary sclerosing cholangitis: fact or fiction? Gut 2001;48:430–4. 35. Shapiro BD, Lashner BA. Cancer biology in ulcerative colitis and potential use in endoscopic surveillance. Gastrointest Endosc Clin N Am 1997;7:453–68. 36. Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet 1994;343:71–4. 37. Ullman TA, Loftus EV, Kakar S, Burgart LJ, Sandborn WJ, Tremaine WJ. The fate of low grade dysplasia in ulcerative colitis. Am J Gastroenterol 2002;97:922–7. 38. Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology 1994;107:934–44. 39. Befrits R, Ljung T, Jaramillo E, Rubio C. Low-grade dysplasia in extensive, long-standing inflammatory bowel disease: a follow-up study. Dis Colon Rectum 2002;45:615–20. 40. Dixon MF, Brown LJ, Gilmour HM, et al. Observer variation in the assessment of dysplasia in ulcerative colitis. Histopathology 1988;13:385–97. 41. Connell WR, Talbot IC, Harpaz N, et al. Clinicopathological characteristics of colorectal carcinoma complicating ulcerative colitis. Gut 1994;35:1419–23. 42. Eaden JA, Abrams K, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35. 43. Odze RD, Goldblum J, Noffsinger A, Alsaigh N, Rybicki LA, Fogt F. Interobserver variability in the diagnosis of ulcerative colitis-associated dysplasia by telepathology. Mod Pathol 2002;15:379–86. 44. Medlicott SA, Jewell LD, Price L, Fedorak RN, Sherbaniuk RW, Urbanski SJ. Conservative management of small adenomata in ulcerative colitis. Am J Gastroenterol 1997;92:2094–8. 45. Rubin PH, Friedman S, Harpaz N, et al. Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology 1999;117:1295–300. 46. Odze RD, Farraye FA, Hecht JL, Hornick JL. Long-term follow-up after polypectomy treatment for adenomalike dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol 2004;2:534–41. 47. Pinczowski D, Ekbom A, Baron J, Yuen J, Adami HO. Risk factors for colorectal cancer in patients with ulcerative colitis: a case-control study. Gastroenterology 1994;107:117–20. 48. Moody GA, Jayanthi V, Probert CS, Mac Kay H, Mayberry JF. Long-term therapy with sulfasalazine protects against colorectal cancer in ulcerative colitis: a retrospective study of colorectal cancer risk and compliance with treatment in Leicestershire. Eur J Gastroenterol Hepatol 1996;8:1179–83. 49. Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Therap 2000; 14:145–53. 50. Gumaste V, Sachar DB, Greenstein AJ. Benign and malignant strictures in ulcerative colitis. Gut 1992;33: 938–41. 51. Lashner BA, Turner BC, Bostwick DG, Frank PH, Hanauer SB. Dysplasia and cancer complicating strictures in ulcerative colitis. Dig Dis Sci 1990;35:349–52. 52. Reiser JR, Waye JD, Janowitz HD, Harpaz N. Adenocarcinoma in strictures of ulcerative colitis without antecedent dysplasia by colonoscopy. Am J Gastroenterol 1994;89:119–22. 53. Alves A, Panis Y, Bouhnik Y, Maylin V, Lavergne-Slove A, Valleur P. Subtotal colectomy for severe acute colitis: a 20-year experience of a tertiary care center with an aggressive and early surgical policy. J Am Coll Surg 2003;197:379–85. 54. Marcello PW, Milsom JW, Wong SK, Brady K, Goormastic M, Fazio VW. Laparoscopic total colectomy for acute colitis: a case-control study. Dis Colon Rectum 2001;44:1441–5. 55. Bell RL, Seymour NE. Laparoscopic treatment of fulminant colitis. Surg Endosc 2002;16:1778–82. 56. Carter FM, McLeod RS, Cohen Z. Subtotal colectomy for ulcerative colitis: complications related to the rectal remnant. Dis Colon Rectum 1991;34:1005–9. 57. Karch LA, Bauer JJ, Gorfine SR, Gelernt IM. Subtotal colectomy with Hartmann’s pouch for inflammatory bowel disease. Dis Colon Rectum 1995;38:635–9. 58. Goligher JC, Hoffman DC, deDombal FT. Surgical treatment of severe attacks of ulcerative colitis. BMJ 1970;4:703–6. 59. Hulten L. Proctocolectomy and ileostomy to pouch surgery for ulcerative colitis. World J Surg 1998;22: 335–41. 60. Jimmo B, Hyman NH. Is ileal pouch-anal anastomosis really the procedure of choice for patients with ulcerative colitis. Dis Colon Rectum 1998;41:41–5. 61. Fazio VW, Tekkis PP, Remzi F, et al. Quantification of risk for pouch failure after ileal pouch anal anastomosis surgery. Ann Surg 2003;238:605–14. Vol. 48, No. 11 PRACTICE PARAMETERS FOR ULCERATIVE COLITIS 2005 62. Carlstedt A, Fasth S, Hulten L, Nordgren S, Palselius I. Long-term ileostomy complications in patients with ulcerative colitis and Crohn’s disease. Int J Colorectal Dis 1987;2:22–5. 63. Camilleri-Brennan J, Munro A, Steele RJ. Does an ileoanal pouch offer a better quality of life than a permanent ileostomy for patients with ulcerative colitis? J Gastrointest Surg 2003;7:814–9. 64. Wikland M, Jansson I, Asztely M, et al. Gynaecological problems related to anatomical changes after conventional proctocolectomy and ileostomy. Int J Colorectal Dis 1990;5:49–52. 65. Hahnloser D, Pemberton JH, Wolff BG, Larson DR, Crownhart BS, Dozois RR. The effect of ageing on function and quality of life in ileal pouch patients: a single cohort experience of 409 patients with chronic ulcerative colitis. Ann Surg 2004;240:615–21. 66. McIntyre PB, Pemberton JH, Wolff BG, Beart RW, Dozois RR. Comparing functional results one year and ten years after ileal pouch-anal anastomosis for chronic ulcerative colitis. Dis Colon Rectum 1994;37: 303–7. 67. Fazio VW, Ziv Y, Church JM, et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg 1995;222:120–7. 68. Meagher AP, Farouk R, Dozois RR, Kelly KA, Pemberton JH. J ileal pouch-anal anastomosis for chronic ulcerative colitis: complications and long-term outcome in 1310 patients. Br J Surg 1998;85:800–18. 69. Martin A, Dinca M, Leone L, et al. Quality of life after proctocolectomy and ileo-anal anastomosis for severe ulcerative colitis. Am J Gastroenterol 1998;93:166–9. 70. Tiainen J, Matikainen M. Health-related quality of life after ileal J-pouch-anal anastomosis for ulcerative colitis: long-term results. Scand J Gastroenterol 1999;34: 601–5. 71. Carmon E, Keidar A, Ravid A, Goldman G, Rabau M. The correlation between quality of life and functional outcome in ulcerative colitis patients after proctocolectomy ileal pouch anal anastomosis. Colorectal Dis 2003;5:228–32. 72. Scarpa M, Angriman I, Ruffolo C, et al. Health-related quality of life after restorative proctocolectomy for ulcerative colitis: long-term results. World J Surg 2004; 28:124–9. 73. Ording Olsen K, Juul S, Berndtsson I, Oresland T, Laurberg S. Ulcerative colitis: female fecundity before diagnosis, during disease, and after surgery compared with a population sample. Gastroenterology 2002;122: 15–9. 74. Johnson P, Richard C, Ravid A, et al. Female infertility after ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum 2004;47:1119–26. 75. Gorgun E, Remzi FH, Goldberg JM, et al. Fertility is reduced after restorative proctocolectomy with ileal pouch anal anastomosis: a study of 300 patients. Surgery 2004;136:795–803. 76. Araki Y, Ishibashi N, Ogata Y, Shirouzu K, Isomoto H. The usefulness of restorative laparoscopic-assisted total colectomy for ulcerative colitis. Kurume Med J 2001;48:99–103. 77. Ky AJ, Sonoda T, Milsom JW. One-stage laparoscopic restorative proctocolectomy: an alternative to the conventional approach? Dis Colon Rectum 2002;45: 207–11. 78. Hasegawa H, Watanabe M, Baba H, Nishibori H, Kitajima M. Laparoscopic restorative proctocolectomy for patients with ulcerative colitis. J Laparoendosc Adv Surg Tech A 2002;12:403–6. 79. Pace DE, Seshadri PA, Chiasson PM, Poulin EC, Schlachta CM, Mamazza J. Early experience with laparoscopic ileal pouch-anal anastomosis for ulcerative colitis. Surg Laparosc Endosc Percutan Tech 2002;12: 337–41. 80. Maartense S, Dunker MS, Slors JF, et al. Hand-assisted laparoscopic versus open restorative proctocolectomy with ileal pouch-anal anastomosis: a randomized trial. Ann Surg 2004;240:984–91. 81. Kienle P, Zgraggen K, Schmidt J, Benner A, Weitz J, Buchler MW. Laparoscopic restorative proctocolectomy. Br J Surg 2005;92:88–93. 82. Taylor BA, Wolff BG, Dozois RR. Ileal pouch-anal anastomosis for chronic ulcerative colitis and familial polyposis coli complicated by adenocarcinoma. Dis Colon Rectum 1988;31:358–62. 83. Radice E, Nelson H, Devine RM, et al. Ileal pouch-anal anastomosis in patients with colorectal cancer: longterm functional and oncologic outcomes. Dis Colon Rectum 1998;41:11–7. 84. Ziv Y, Fazio VW, Strong SA, Oakley JR, Milsom JW, Lavery IC. Ulcerative colitis and coexisting colorectal cancer: recurrence rate after restorative proctocolectomy. Ann Surg Oncol 1994;1:512–5. 85. Gorfine SR, Harris MT, Bub DS, Bauer JJ. Restorative proctocolectomy for ulcerative colitis complicated by colorectal cancer. Dis Colon Rectum 2004;47:1377– 385. 86. Stelzner M, Fonkalsrud EW. The endorectal ileal pullthrough procedure in patients with ulcerative colitis and familial polyposis with carcinoma. Surg Gynecol Obstet 1989;169:187–94. 87. Wiltz O, Hashmi HF, Schoetz DJ Jr, et al. Carcinoma and the ileal pouch-anal anastomosis. Dis Colon Rectum 1991;34:805–9. 88. Tan HT, Connolly AB, Morton D, Keighley MR. Results of restorative proctocolectomy in the elderly. Int J Colorectal Dis 1997;12:319–22. 89. Takao Y, Gilliland R, Nogueras JJ, Weiss EG, Wexner SD. Is age relevant to functional outcome after restorative proctocolectomy for ulcerative colitis? Prospec- 2006 COHEN ET AL Dis Colon Rectum, November 2005 tive assessment of 122 cases. Ann Surg 1998;227: 187–94. 90. Delaney CP, Fazio VW, Remzi FH, et al. Prospective, age-related analysis of surgical results, functional outcome, and quality of life after ileal pouch-anal anastomosis. Ann Surg 2003;238:221–8. 91. Longo WE, Virgo KS, Bahadursingh AN, Johnson FE. Patterns of disease and surgical treatment among United States Veterans more than 50 years of age with ulcerative colitis. Am J Surg 2003;186:514–8. 92. Michelassi F, Lee J, Rubin M, et al. Long-term functional results after ileal pouch anal restorative proctocolectomy for ulcerative colitis: a prospective observational study. Ann Surg 2003;238:433–41. 93. Bullard KM, Madoff RD, Gemlo BT. Is ileoanal pouch function stable with time? Dis Colon Rectum 2002;45: 299–304. 94. Lavery IC, Sirimarco MT, Ziv Y, Fazio VW. Anal canal inflammation after ileal pouch-anal anastomosis: the need for treatment. Dis Colon Rectum 1995;38:803–6. 95. Shen B, Lashner BA, Bennett AE, et al. Treatment of rectal cuff inflammation (cuffitis) in patients with ulcerative colitis following restorative proctocolectomy and ileal pouch-anal anastomosis. Am J Gastroenterol 2004;99:1527–31. 96. Sequens R. Cancer in the anal canal (transitional zone) after restorative proctocolectomy with stapled ileal pouch-anal anastomosis. Int J Colorectal Dis 1997;12: 254–5. 97. Rotholtz NA, Pikarsky AJ, Singh JJ, Wexner SD. Adenocarcinoma arising from along the rectal stump after double-stapled ileorectal J-pouch in a patient with ulcerative colitis: the need to perform a distal anastomosis. Dis Colon Rectum 2001;44:1214–7. 98. Baratsis S, Hadjidimitriou F, Christodoulou M, Lariou K. Adenocarcinoma in the anal canal following ileal pouch-anal anastomosis for ulcerative colitis using a double stapling technique. Dis Colon Rectum 2002;45: 687–92. 99. Seow-Choen A, Tsunoda A, Nicholls RJ. Prospective randomized trial comparing anal function after handsewn ileoanal anastomosis versus stapled ileoanal anastomosis without mucosectomy in restorative proctocolectomy. Br J Surg 1991;78:430–4. 100. Luukkonen P, Jarvinen H. Stapled versus hand sutured ileoanal anastomosis in restorative proctocolectomy: a prospective randomized trial. Arch Surg 1993;128: 437–40. 101. Reilly WT, Pemberton JH, Wolff BG, et al. Randomized prospective trial comparing ileal pouch-anal anastomosis performed by excising the anal mucosa to ileal pouch-anal anastomosis. Ann Surg 1997;225:666–76. 102. O’Riordain MG, Fazio VW, Lavery IC, et al. Incidence and natural history of dysplasia of the anal transitional zone after ileal pouch-anal anastomosis: results of a five-year to ten-year follow-up. Dis Colon Rectum 2000;43:1660–5. 103. Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. BMJ 1978;2:85–8. 104. Utsunomiya J, Iwama T, Imago M, et al. Total colectomy, mucosal proctectomy and ileoanal anastomosis. Dis Colon Rectum 1980;23:459–66. 105. Fonkalsrud EW. Total colectomy and endorectal ileal pull-through with internal ileal reservoir for ulcerative colitis. Surg Gynecol Obstet 1980;150:1–8. 106. Nicholls RJ, Lubowski DZ. Restorative proctocolectomy: the four loop (W) reservoir. Br J Surg 1987;74: 546–66. 107. Rothenberger DA, Buls JG, Nivatvongs S, Goldberg SM. The Parks S ileal pouch and anal anastomosis after colectomy and mucosal proctectomy. Am J Surg 1985; 149:390–4. 108. Stone MM, Lewin K, Fonkalsrud EW. Late obstruction of the lateral ileal reservoir after colectomy and endorectal ileal pullthrough procedures. Surg Gynecol Obstet 1986;162:411–7. 109. Johnston D, Williamson ME, Lewis WG, Miller AS, Sagar PM, Holdsworth PJ. Prospective controlled trial of duplicated (J) versus quadrupled (W) pelvic ileal reservoirs in restorative proctocolectomy for ulcerative colitis. Gut 1996;39:242–7. 110. Keighley MR, Yoshioka K, Kmiot W. Prospective randomized trial to compare the stapled double lumen pouch and the sutured quadruple pouch for restorative proctocolectomy. Br J Surg 1998;75:1008–11. 111. Smith L, Friend WG, Medwell SJ. The superior mesenteric artery. The critical factor in the pouch pullthrough procedure. Dis Colon Rectum 1984;27:741–4. 112. Tjandra JJ, Fazio VW, Milsom JW, Lavery IC, Oakley JR, Fabre JM. Omission of temporary diversion in restorative proctocolectomy: is it safe? Dis Colon Rectum 1993;36:1007–14. 113. Everett WG, Pollard SG. Restorative proctocolectomy without temporary ileostomy. Br J Surg 1990;77:621–2. 114. Galandiuk S, Wolff BG, Dozois RR, Beart RW Jr. Ileal pouch-anal anastomosis without ileostomy. Dis Colon Rectum 1991;34:870–3. 115. Järvinen HJ, Luukkonen P. Comparison of restorative proctocolectomy with and without covering ileostomy in ulcerative colitis. Br J Surg 1991;78:199–201. 116. Cohen Z, McLeod RS, Stephen W, Stern HS, O’Connor B, Reznick R. Continuing evolution of the pelvic pouch procedure. Ann Surg 1992;216:506–11. 117. Grobler SP, Hosie KB, Keighley MR. Randomized trial of loop ileostomy in restorative proctocolectomy. Br J Surg 1992;79:903–6. 118. Sugerman HJ, Newsome HH Jr. Stapled ileo-anal anastomosis without a temporary ileostomy. Am J Surg 1994;167:58–66. 119. Gorfine SR, Gelernt IM, Bauer JJ, Harris MT, Kreel I. Vol. 48, No. 11 PRACTICE PARAMETERS FOR ULCERATIVE COLITIS 2007 Restorative proctocolectomy without diverting ileostomy. Dis Colon Rectum 1995;38:188–94. 120. Williamson ME, Lewis WG, Sagar PM, Holdsworth PJ, Johnston D. One-stage restorative proctocolectomy without temporary ileostomy for ulcerative colitis. Dis Colon Rectum 1997;40:1019–22. 121. Heuschen UA, Allenmeyer EH, Hinz U, Lucas M, Herfarth C, Heuschen G. Outcome after septic complications in J pouch procedures. Br J Surg 2002;89:194– 200. 122. Sugerman HJ, Sugerman EL, Meador JG, Newsome HH Jr, Kellum JM Jr, DeMaria EJ. Ileal pouch anal anastomosis without ileal diversion. Ann Surg 2000;232:530– 41. 123. Farouk R, Dozois RR, Pemberton JH, Larson D. Incidence and subsequent impact of pelvic abscess after ileal pouch-anal anastomosis for chronic ulcerative colitis. Dis Colon Rectum 1998;41:1239–43. 124. Mowschenson PM, Critchlow JF, Peppercorn MA. Ileoanal pouch operation: long-term outcome with or without diverting ileostomy. Arch Surg 2000;135: 463–5. 125. Gullberg K, Liljeqvist L. Stapled ileoanal pouches without loop ileostomy: a prospective study in 86 patients. Int J Colorectal Dis 2001;16:221–7. 126. MacLean AR, Cohen Z, MacRae HM, et al. Risk of small bowel obstruction after the ileal pouch-anal anastomosis. Ann Surg 2002;235:200–6. 127. Gunnarsson U, Karlbom U, Docker M, Raab Y, Pahlman L. Proctocolectomy and pelvic pouch—is a diverting stoma dangerous for the patient? Colorectal Dis 2004;6:23–7. 128. Heuschen UA, Hinz U, Allemeyer EH, Lucas M, Heuschen G, Herfarth C. One- or two-stage procedure for restorative proctocolectomy: rationale for a surgical strategy in ulcerative colitis. Ann Surg 2001;234:788– 94. 129. Setti-Carraro P, Talbot IC, Nicholls RJ. Long-term appraisal of the histological appearances of the ileal reservoir mucosa after restorative proctocolectomy for ulcerative colitis. Gut 1994;35:1721–7. 130. Fruin AB, El-Zammer O, Stucchi AF, O’Brien M, Becker JM. Colonic metaplasia in the ileal pouch is associated with inflammation and is not the result of long-term adaptation. J Gastrointest Surg 2003;7:246– 53. 131. Herline AJ, Meisinger LL, Rusin LC, et al. Is routine pouch surveillance for dysplasia indicated for ileoanal pouches? Dis Colon Rectum 2003;46:156–9. 132. Thompson-Fawcett MW, Marcus V, Redston M, Cohen Z, McLeod RS. Risk of dysplasia in long-term ileal pouches and pouches with chronic pouchitis. Gastroenterology 2001;121:275–81. 133. Borjesson L, Willen R, Haboubi N, Duff SE, Hulten L. The risk of dysplasia and cancer in the ileal pouch mucosa after restorative proctocolectomy for ulcerative proctocolitis is low: a long-term term follow-up study. Colorectal Dis 2004;6:494–8. 134. Lohmuller JL, Pemberton JH, Dozois RR, Ilstrup D, van Heerden J. Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch-anal anastomosis. Ann Surg 1990;211:622–7. 135. Penna C, Dozois R, Tremaine W, et al. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut 1996;38: 234–9. 136. Gustavsson S, Weiland LH, Kelly KA. Relationship of backwash ileitis to ileal pouchitis after ileal pouch-anal anastomosis. Dis Colon Rectum 1987;30:25–8. 137. Samarasekera DN, Stebbing JF, Kettlewell MG, Jewell DP, Mortensen NJ. Outcome of restorative proctocolectomy with ileal reservoir for ulcerative colitis: comparison of distal colitis with more proximal disease. Gut 1996;38:574–7. 138. Schmidt CM, Lazenby AJ, Hendrickson RJ, Sitzmann JV. Preoperative terminal ileal and colonic resection histopathology predicts risk of pouchitis in patients after ileoanal pull-through procedure. Ann Surg 1998; 227:663–5. 139. Campieri M, Gionchetti P. Probiotics in inflammatory bowel disease: new insight to pathogenesis or a possible therapeutic alternative? Gastroenterology 1999; 116:1246–9. 140. Mahadevan U, Sandborn WJ. Diagnosis and management of pouchitis. Gastroenterology 2003;124:1636– 50. 141. Shen B, Achkar JP, Lashner BA, et al. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology 2001;121:261–7. 142. Shen B, Achkar JP, Conor JT, et al. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum 2003;46:748– 53. 143. Madden MV, McIntyre AS, Nicholls RJ. Double-blind crossover trial of metronidazole versus placebo in chronic unremitting pouchitis. Dig Dis Sci 1994;39: 1193–6. 144. Sandborn WJ, Pardi DS. Clinical management of pouchitis. Gastroenterology 2004;127:1809–14. 145. Shen B, Achkar JP, Lashner BA, et al. A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm Bowel Dis 2001;7: 301–5. 146. Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a doubleblind, placebo-controlled trial. Gastroenterology 2003; 124:1202–9. 147. Gionchetti P, Rizzello F, Venturi A, et al. Maintenance 2008 COHEN ET AL Dis Colon Rectum, November 2005 treatment of chronic pouchitis: a randomized placebocontrolled, double-blind trial with a new probiotic preparation. Gastroenterology 2000;119:305–9. 148. Sambuelli A, Boerr L, Negreira S, et al. Budesonide enema in pouchitis—a double-blind, double-dummy, controlled trial. Aliment Pharmacol Therap 2002;16: 27–34. 149. Hulten L, Fasth S, Hallgren T, Oresland T. The failing pelvic pouch: conversion to continent ileostomy. Int J Colorectal Dis 1992;7:119–21. 150. Ecker KW, Hildebrandt U, Haberer M, Feilfel G. Biomechanical stabilization of the nipple valve in continent ileostomy. Br J Surg 1996;83:1582–5. 151. Lepisto AH, Jarvinen HJ. Durability of the Kock continent ileostomy. Dis Colon Rectum 2003;46:925–8. 152. Vernava AM, Goldberg SM. Is the Kock pouch still a viable option? Int J Colorectal Dis 1988;3:135–8. 153. Fazio VW, Church JM. Complications and function of the continent ileostomy at the Cleveland Clinic. World J Surg 1988;12:148–54. 154. Fazio VW, Tjandra JJ. Technique for nipple valve fixation to prevent valve slippage in continent ileostomy. Dis Colon Rectum 1992;35:1177–9. 155. Little VR, Barbour S, Schrock TR, Welton ML. The continent ileostomy: long-term durability and patient satisfaction. J Gastrointest Surg 1999;3:625–32. 156. Ojerskog B, Hallstrom T, Kock NG, Myrvold HE. Quality of life in ileostomy patients before and after conversion to the continent ileostomy. Int J Colorectal Dis 1988;3:166–70. 157. Parc R, Legrand M, Frileux P, Tiret E, Ratelle R. Comparative clinical results of ileal-pouch anal anastomosis and ileorectal anastomosis in ulcerative colitis. Hepatogastroenterology 1989;36:235–9. 158. Khubchandani IT, Kontostolis SB. Outcome of ileorectal anastomosis in an inflammatory bowel disease surgery experience of three decades. Arch Surg 1994;129: 866–9. 159. Pastore RL, Wolff BG, Hodge D. Total abdominal colectomy and ileorectal anastomosis for inflammatory bowel disease. Dis Colon Rectum 1977;40:1455–64. 160. Saito Y, Sawada T, Tsuno A, et al. Total colectomy and ileorectal anastomosis in ulcerative colitis. J Gastroenterol 1995;8:131–4. 161. Kvist N, Jacobsen O, Kvist HK, et al. Malignancy in ulcerative colitis. Scand J Gastroenterol 1989;24:497– 506. 162. Leijonmarck CE, Lofberg R, Ost A, Hellers G. Longterm results of ileorectal anastomosis in ulcerative colitis in Stockholm County. Dis Colon Rectum 1990;33: 195–200. 163. Lofberg R, Leijonmarck CE, Brostrom O. Mucosal dysplasia and DNA content in ulcerative colitis patients with ileorectal anastomosis. Follow-up study in a definite patient group. Dis Colon Rectum 1991;34:566–71. 164. Gruner OP, Flatmark A, Naas R, Fretheim B, Gjone E. Ileorectal anastomosis in ulcerative colitis. Results in 57 patients. Scand J Gastroenterol 1975;10:641–6. 165. Paoluzi OA, Di Paolo MC, Ricci F, Pasquali C, Iacucci M, Paoluzi P. Ileo-rectal anastomosis in ulcerative colitis: results of a long-term follow-up study. Ital J Gastroenterol 1994;26:392–7