Practice Parameters for the Management of Colon Cancer
George J. Chang, M.D. • Andreas M. Kaiser, M.D. • Steven Mills, M.D.
Janice F. Rafferty, M.D. • W. Donald Buie, M.D., on behalf of the Standards Practice Task Force of the American Society of Colon and Rectal Surgeons
The American Society of Colon and Rectal Surgeons is dedicated to ensuring high quality patient care by advancing the science, prevention, and management of disorders and diseases of the colon, rectum, and anus. The Standards Committee is composed of Society members who are chosen because they have demonstrated expertise in the specialty of colon and rectal surgery. This Committee was created to lead international efforts in defining quality care for conditions related to the colon, rectum, and anus. This is accompanied by developing Clinical Practice Guidelines based on the best available evidence. These guidelines are inclusive, and not prescriptive. Their purpose is to provide information on which to base decisions, rather than dictate a specific form of treatment. These guidelines are intended for the use of all practitioners, health care workers, and patients who desire information about the management of the conditions addressed by the topics covered in these guidelines. It should be recognized that these guidelines should not be deemed inclusive of all proper methods of care or exclusive of methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding the propriety of any specific procedure must be made by the physician in light of all of the circumstances presented by the individual patient.
STATEMENT OF THE PROBLEM
Colon cancer affects approximately 107,000 new patients in the United States each year and is the third leading cause of cancer death among men and women. Despite significant improvements in prevention and treatment of colon cancer over the past several years, in 2010 approximately 30,000 deaths are estimated to have been due to colon cancer.1 Most patients will present with localized disease amenable to curative surgical resection, but approximately 20% of patients will still present with distant metastases. The treatment of patients with colon cancer is largely guided by stage at presentation, emphasizing the importance of a comprehensive strategy of diagnosis, evaluation, and treatment.
The scope of this parameter will be to address the issues related to the evaluation and treatment of patients who have been diagnosed with colon cancer. Issues pertinent to colon cancer screening and surveillance after colon cancer treatment as well as rectal cancer will be addressed in separate documents.
METHODS
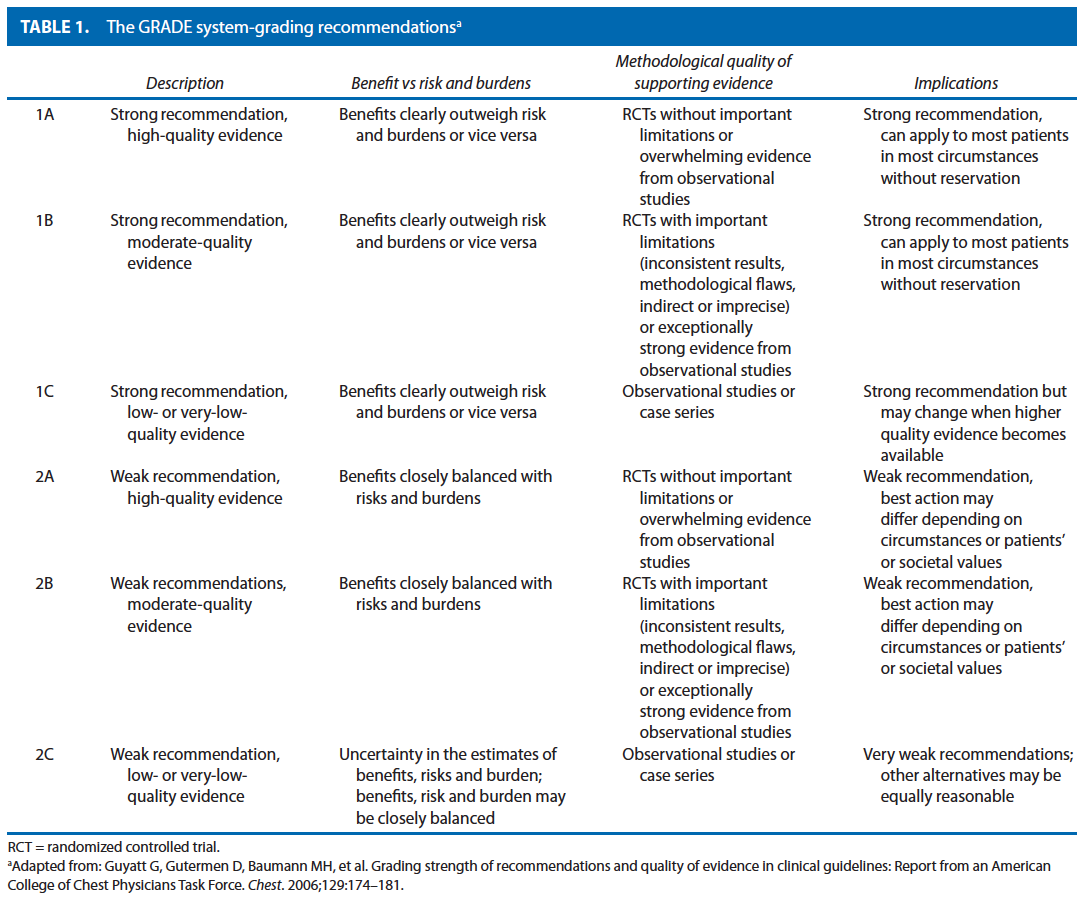
This parameter is based on the previous parameter published in 2004. An organized search of MEDLINE, PubMed, Embase, and the Cochrane Database of Collected Reviews was performed through February 2010. Key word combinations included colonic or colorectal neoplasms, adenocarcinoma, chemotherapy, colonoscopy, staging, lymph node, neoplasm metastasis, peritoneal neoplasm, surgical procedures, and recurrence. Directed searches of the embedded references from the primary articles were also performed in selected circumstances. All English language articles and studies of adults were reviewed by the primary authors. In selected instances where a full article was not yet available, reports of conference proceedings were reviewed. Recommendations were formulated by the primary authors and reviewed by the entire Standards Committee. The final grade of recommendation was performed by using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) system (Table 1) and reviewed by the entire Standards Committee.
EVALUATION AND RISK ASSESSMENT
A thorough disease history should be obtained eliciting diseasespecific symptoms, associated symptoms, and family history. Routine laboratory values, including CEA levels, should also be evaluated, as indicated. Grade of Recommendation: 1C
Patients with colon cancer are often asymptomatic. Some have symptoms of change in bowel habits, blood in their stool, anemia, or are found to be fecal occult blood positive. Less often, a patient may have pain or obstructive symptoms or symptoms of metastatic disease. A complete history, including family history and colon cancerspecific history can guide the surgeon to suspect hereditary cancer syndromes, look for associated pathology or metastatic disease, and initiate additional workup such as mutational analysis of the tumor. Patients meeting clinical criteria for or having a family history of an increased susceptibility to colorectal cancer should be referred for genetic counseling for formal evaluation.
Routine laboratory examinations including complete blood cell count, liver function tests, and chemistry panel should be performed, based upon patient comorbidities, as indicated for preparation for general anesthesia.2 Because anemia can be common in patients with colon cancer, a complete blood count including platelets is recommended before surgical intervention. Carcinoembryonic antigen levels should be assessed before elective surgery for colon cancer for the establishment of baseline values and during the surveillance period to monitor for signs of recurrence.3 Although higher levels of CEA have been correlated with poorer prognosis, the data are insufficient to justify the use of a high preoperative CEA as an indication for adjuvant therapy.4,5 A confirmed rise in the postoperative CEA during surveillance should prompt further investigation for recurrent disease.6 At present there is insufficient evidence to support the routine use of other tumor markers such as CA199 in the routine evaluation of patients with colon cancer.4
When possible, all patients with presumed or proven colon cancer should undergo a full colonic evaluation with histologic assessment of the colonic lesion before treatment. Grade of Recommendation: 1C
Although the majority of patients are diagnosed with colon cancer during a full colonoscopy, new screening recommendations have recently been published that give alternatives to endoluminal examination.7–10 An increasing number of patients may be diagnosed by these newer methods and may be referred for surgical therapy without having previously undergone complete endoluminal examination with histologic tissue diagnosis. In cases without documented complete intracolonic evaluation, full endoluminal examination with biopsy is advocated. Whenever possible, the histologic diagnosis of colon cancer should be confirmed before elective surgical resection because nonneoplastic processes such as diverticulitis or IBD may be associated with the endoscopic appearance of colon cancer.
The risk for synchronous carcinomas or adenomas within the colon may be as high as 10% in the general population. Preoperative evaluation and diagnosis allow the surgeon to diagnose and potentially treat other colonic polyps, or, in the case of a synchronous cancer, choose the correct extent of colonic resection. The identification of synchronous cancers may also lead to workup for underlying predisposing risk factors such as inheritable colorectal cancer syndromes. In addition, endoscopic marking of the lesion location (tattoo) could be performed, especially in cases where laparoscopic resection is planned.
Some patients undergo colonoscopy, but the examination cannot be completed. In the absence of a clinically complete obstruction or perforation, a radiological study should be obtained to complete the colonic evaluation. These include contrast enemas (eg, barium enema) or preferably CT colonography or PET/CT colonography.11–13 In circumstances where the examination could not be completed but the patient meets indications for adjuvant chemotherapy, the completion colonoscopy should be performed soon after completion of chemotherapy.
Preoperative radiologicalo staging should be routinely performed. Grade of Recommendation: 1B
Preoperative radiographic staging including a CT scan of the chest, abdomen, and pelvis should be routinely performed before the elective surgical resection of colon cancer. This permits the detection and evaluation of the extent of synchronous metastases that may require a change in the treatment strategy, eg, chemotherapy rather than surgery first or potential simultaneous resection of both the primary tumor and the metastatic sites. The preoperative CT scan findings may also result in the operative plan being altered based on identification of the tumor location, adjacent organ or abdominal wall involvement, in addition to the presence of metastatic disease. In the event that a patient has been referred with CT imaging only of the abdomen and pelvis, at minimum, a preoperative chest xray should be obtained and a CT of the chest should be performed postoperatively. In patients with hypersensitivity to the iodine contrast dye, or in the appropriate clinical setting to work up indeterminate lesions on CT, an 18FDGPET fused CT scan or noncontrast chest CT with an MRI of the abdomen and pelvis may be considered. In some situations, a preoperative or intraoperative ultrasound may provide additional information.
The importance of preoperative imaging is also supported by practice guidelines of a number of national and international organizations, including the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the Association of Coloproctology of Great Britain and Ireland, the European Society of Medical Oncology, and others.3,14,15
STAGING OF COLON CANCER
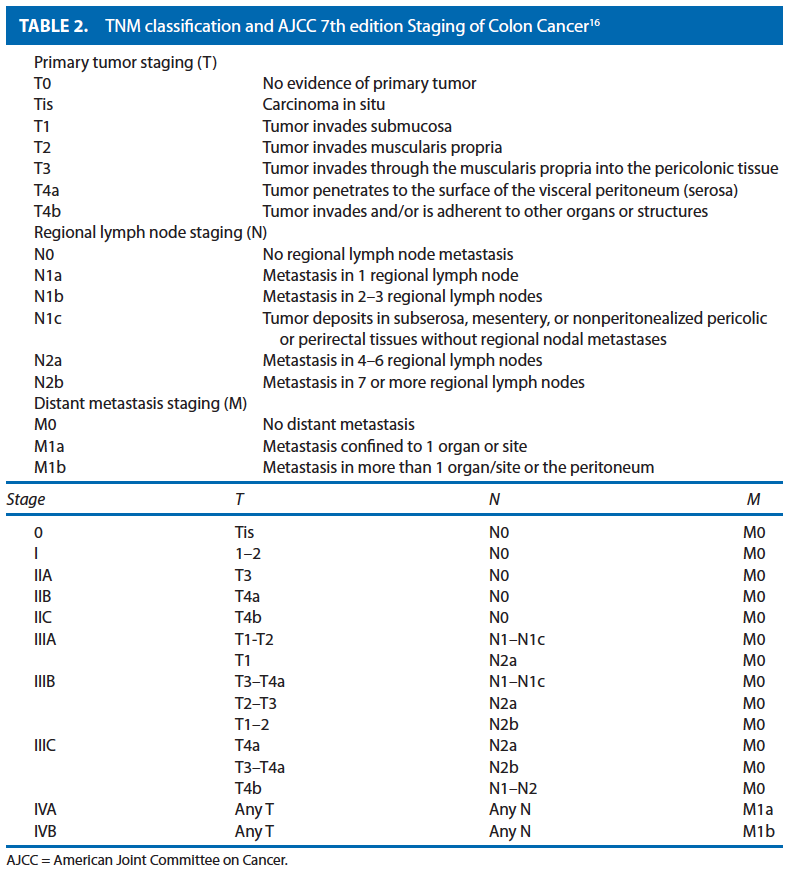
Colon cancer staging should be performed according to the American Joint Committee on Cancer (AJCC)/TNM system and include an assessment of the completeness of surgical resection designated by the residual tumor code “R.” Grade of Recommendation: 1B
The tumor depth, nodal metastasis, and distant metastasis have been shown to be predictors of prognosis in colon cancer. These characteristics are described by the Union for International Cancer Control/AJCC TNM staging system, initially described in 2002, but that recently has been updated with the 7th edition of the AJCC Cancer Staging Manual16 and is presented in Table 1. The new system is based on observed survival outcomes within the US populationbased Surveillance Epidemiology and End Results cancer registry.17 Further stratification of tumor deposits and types of metastases to reflect the continuously evolving treatment of patients with advanced disease has been incorporated into the new edition of the staging system.18 In addition, prognostic calculators and nomograms have been proposed and may be considered in the future.19,20
In addition to TNM staging, the histologic grade of the tumor as well as the completeness of the resection should be assessed. Histologic grade has also been shown to be an important predictor of outcome and is an important consideration for treatment recommendations. The absence or presence of residual tumor following resection is designated by the letter R in accordance with the AJCC prognostic factors, as indicated below, and where possible should be indicated in the operative report:
• R0—complete tumor resection with all margins histologically negative
• R1—incomplete tumor resection with microscopic surgical resection margin involvement (margins grossly uninvolved)
• R2—incomplete tumor resection with gross residual tumor that was not resected (primary tumor, regional nodes, macroscopic margin involvement).21
SURGICAL TREATMENT OF THE PRIMARY TUMOR
The primary treatment for localized resectable colon cancer is colectomy with en bloc removal of all associated regional lymph nodes and involved adjacent structures. A thorough exploration should be performed at the time of resection. The value of the “no touch” technique in which the vascular supply to and from the tumor are divided before manipulating the tumor has remained controversial, and definite benefit has not been demonstrated.22,23 However a principle of gentle handling of the tumor during operation should be observed to avoid the risk for tumor spillage or perforation, in particular, in the management of locally advanced tumors or those with associated abscess.
a. A thorough surgical exploration should be performed and the findings documented in the operative report. Grade of Recommendation: 1C
The surgical exploration includes a visual or palpatory assessment of the peritoneal cavity and the abdominal organs to detect or rule out synchronous lesions, more advanced malignant disease (carcinomatosis, adjacent organ involvement, occult metastasis), or coexisting pathology (eg, adhesions, hernia, cholelithiasis, cirrhosis, etc.).14,21
b. The extent of resection of the colon should correspond to the lymphovascular drainage of the site of the colon cancer. The lymphadenectomy should be complete and en bloc with the bowel segment. Grade of Recommendation: 1A
The extent of a curative resection for colon cancer depends on (1) the site of the primary lesion and (2) the lymphovascular drainage of the cancer site. The length of bowel resected is governed by the blood supply to that segment. In the absence of synchronous pathology, an anatomic colon resection for cancer should achieve at least a 5cm negative margin on either side of the tumor. A colotomy and local excision of a colon cancer is not an adequate surgical technique for curative resection. It is associated with a risk of tumor spillage into the peritoneal cavity, and the lack of a lymphadenectomy increases the risk of tumor progression.3,14,21
The mesentery to the tumorbearing segment of bowel should be removed to the origin of the named primary feeding vessel(s). This resection should be performed en bloc with preservation of the integrity of the colonic mesentery.24,25 In the absence of clinical involvement, a more radical resection above the primary feeding vessel has not been associated with improved survival (eg, superior mesenteric versus ileocolic lymph node dissection for ascending colon cancer). The complete surgical removal of the regional lymph nodes within the mesocolon allows for a curative resection and accurate pathologic staging of the disease. When suspected to be involved, the most apical lymph nodes should be marked on the specimen as their metastatic involvement is a negative prognostic indicator. Because the total number of lymph nodes evaluated at the time of resection has been associated with survival, the lymph node examination should be as complete as possible.26,27 It is recommended that at least 12 lymph nodes be evaluated to assign N0 stage, and the examination of fewer than 12 lymph nodes is a highrisk feature for stage II colon cancer.3 In the event that fewer than 12 lymph nodes are reported on the pathology report, the surgeon is encouraged to request processing and reporting of the specimen in accordance to the guidelines set forth by the College of American Pathologists.28,29
c. Clinically positive lymph nodes located outside the standard field of resection identified at the time of resection and suspected to contain metastatic disease should be biopsied or removed at the time of primary resection. Grade of Recommendation: 2B
If residual tumorbearing lymph nodes remain following sampling, the resection will be considered incomplete. If no residual tumorbearing lymph nodes remain, the resection may be considered complete.21 High ligation is defined by an extended lymphadenectomy beyond the primary lymph node distribution (eg, ligation and resection of inferior mesenteric vessels and lymph nodes at the aorta, rather than superior rectal vessels and lymph nodes at their origin for a distal sigmoid carcinoma). Standard ligation is performed at the origin of the primary feeding vessel and should include all associated lymph nodes. In the absence of clinical evidence for metastasis to the extended lymph node distribution, high ligation beyond the origin of the primary feeding vessel has not been shown to improve survival.30 However, in the context of modern systemic chemotherapy, resection of isolated metastases to retroperitoneal lymph nodes may be considered in the context of a multidisciplinary setting.
d. Resection of involved adjacent organs should be en bloc. Grade of Recommendation: 1B
Local tumor control is achieved by complete resection of the tumor en bloc with contiguously involved structures. It may not be possible to distinguish between inflammatory and malignant adhesions, and peritumoral adhesions have been shown to harbor malignant cells in more than 40% of cases. Therefore, peritumoral adhesions should not be divided, and the adherent structure should be excised en bloc.3,14,21 Available diagnostic modalities (eg, CT scan or MRI scan) should be used to facilitate the identification of adjacent organ involvement before surgical exploration, so that adequate preparation and assembly of a multidisciplinary team may be performed. Local tumor control is achieved by complete resection of the tumor en bloc with contiguously involved structures.31–33 Tumor debulking in the setting of resectable adjacent organ involvement should not be performed.
Synchronous colon cancers can be treated by 2 separate resections or subtotal colectomy. Grade of Evidence: 1B
In recent years, the incidence of synchronous primary colorectal cancers is estimated to be 2% to 5% of all patients presenting with primary colorectal cancer, although the true incidence is probably related to underlying screening intensity.9,34,35 Patients with synchronous tumors should be evaluated for an associated genetic colorectal cancer syndrome and their treatment appropriately tailored. Synchronous pathology (cancer or endoscopically unresectable polyps) may be safely managed by an extended resection incorporating both lesions or 2 separate resections.36
When associated with underlying colonic disease (eg, chronic ulcerative colitis or hereditary nonpolyposis colorectal cancer syndrome), the extent of resection should consider treatment of the underlying disorder. For example, carcinoma arising in the setting of chronic ulcerative colitis should be treated with a proctocolectomy, whereas carcinoma arising in the setting of hereditary nonpolyposis colorectal cancer may be treated by either tumordirected segmental resection or by a more extensive resection tailored to the underlying risk of the patient37,38
Sentinel lymph node (SLN) mapping for colon cancer does not replace standard lymphadenectomy. Grade of Recommendation: 1B
Despite continued interest in SLN mapping and ultrastaging for colon cancer, to date, the results of SLN mapping for staging remain discordant and are not sufficiently accurate for identifying lymph node metastases, with a particular concern for the high rate of falsenegative nodal staging.39–43 A potential benefit of SLN techniques could be to support the pathologist in identifying lymph nodes ex vivo and hence decrease the risk for understaging the tumor. Furthermore, the clinical significance of micrometastatic disease within the SLN (as identified by hematoxylin and eosin staining or immunohistochemical and APC gene polymerase chain reaction analysis) remains undefined.44,45 Immunohistochemical ultrastaging currently does not appear to be clinically relevant, but polymerase chain reaction amplification of tumorrelated genes may show promise.
Laparoscopic and open colectomy achieve equivalent oncological outcomes for localized colon cancer. The use of the laparoscopic approach should be based on the surgeon’s documented experience in laparoscopic surgery as well as on patient and tumorspecific factors. Grade of Recommendation: 1A
The laparoscopic procedure should achieve the same goals as the open approach; a conversion to a laparotomy approach is otherwise recommended. A number of large multiinstitutional randomized trials with experienced participating surgeons in the United States and internationally have demonstrated equivalent overall and recurrencefree survival rates after laparoscopic in comparison with open surgical resection of localized colon cancer, excluding tumors within the rectum or transverse colon. One randomized trial did demonstrate a survival benefit with laparoscopic surgery, but other studies have not reproduced this observation.46 The short and longterm oncological outcomes have also been shown to be equivalent between patients treated with an open or laparoscopic approach.43,47–52 This practice parameter advocates the American Society of Colon and Rectal Surgeons position statement regarding credentialing of surgeons to perform laparoscopic colectomy for cancer.53
Treatment of the malignant polyp is determined by the morphology and histology of the polyp. Grade of Recommendation: 1B
Adenomatous polyps of the colon can be classified to be pedunculated or sessile. A malignant adenomatous polyp is defined as one in which cancer is invading through the muscularis mucosae into the submucosa (T1). It is estimated that 2% to 5% of adenomatous polyps will be associated with invasive malignancy. A number of classification systems have been developed to describe malignant polyps on the basis of which guidance for clinical management can be developed (Haggitt, Sm, Japanese Society for Cancer of the Colon and Rectum, and the Paris endoscopic classification of superficial GI neoplastic lesions).54–56
Endoscopic management can be sufficient for selected malignant polyps, if they are pedunculated or have protruding morphology and are lowrisk lesions with no adverse histologic features. Lowrisk polyps are those that are completely removed, preferably after submucosal elevation, without specimen fragmentation, are not poorly differentiated, and show no lymphatic or vascular invasion, or extension of the tumor to the margin of stalk resection. For lesions meeting lowrisk criteria, endoscopic resection followed by observation is appropriate. Those with adverse histologic features are at a higher risk for nodal metastases and formal oncological resection should be performed after weighing the risk of surgery against the risk of tumor progression.7,8,57
PROPHYLACTIC ONCOLOGICAL RESECTION OF EXTRAINTESTINAL ORGANS
Oophorectomy is advised for grossly abnormal ovaries or contiguous extension of the colon cancer, but routine prophylactic oophorectomy is not necessary. Grade of Recommendation: 1C
The ovaries are the site for colon cancer metastasis in fewer than 15% of patients, but colon cancer metastases to the ovaries can reach a considerable size (Krukenberg tumor). At this time, there are insufficient data to support routine prophylactic oophorectomy at the time of colectomy; however, oophorectomy should be performed during resection of the primary tumor with curative intent in patients suspected or known to have ovarian involvement, either by direct extension or metastasis.58 If 1 ovary is involved with metastatic disease, a bilateral oophorectomy should be performed. Limited data exist regarding prophylactic oophorectomy in women with colon cancer without other risk factors for ovarian pathology such as hereditary nonpolyposis colorectal cancer or BRCA.59 Routine prophylactic oophorectomy of normalappearing ovaries has not been associated with improved survival; however, there are insufficient data to recommend strongly for or against it.54 Oophorectomy may be considered in postmenopausal women after preoperative consultation, or in women at risk for ovarian cancer.
MANAGEMENT OF SYNCHRONOUS STAGE IV DISEASE
Approximately 15% to 20% of patients with have liver or lung metastases at the time of initial presentation with colon cancer. Among these approximately 20% to 25% of patients will have potentially resectable disease.60 The treatment of patients presenting with synchronous stage IV disease should be individualized and guided by a multidisciplinary team of diseasesite surgeons (colorectal surgeon, hepatic and/or thoracic surgeon) and medical oncologist.61,62 Patients may be classified as initially having resectable or potentially resectable disease, and unresectable disease with respect to both their primary tumor site and metastases.
Resectable Stage IV Disease
The treatment of patients with resectable stage IV colon cancer should be individualized based on comprehensive multidisciplinary evaluation. Grade of Recommendation: 1B
The treatment of patients with resectable metastatic colon cancer should be individualized and determined by multidisciplinary consensus. When the metastatic disease is potentially resectable, resection of the primary tumor should be complete and radical consistent with oncological principals of resection for localized disease as previously outlined in this document. In general, medically fit patients with resectable hepatic and/or pulmonary metastases will benefit from curative resection of the metastases.63 The sequence of chemotherapy, resection of the primary tumor, and resection of metastasis should be individualized and determined by multidisciplinary consensus.61,62 The disease of some patients may be converted to resectable after systemic chemotherapy.64 Neoadjuvant approaches to systemic chemotherapy before surgical resection may assist in identifying patients who are candidates for surgical resection.65,66 Patient survival is improved by the addition of systemic chemotherapy to surgical resection.60,67
Unresectable Stage IV Disease
Palliative intervention or resection of the symptomatic primary tumor should be considered, but routine resection of the asymptomatic primary tumor is not recommended. Grade of Recommendation: 1B
Patients with unresectable metastatic disease should be treated with systemic chemotherapy with palliative intent. With sequential therapies, the current median survival among patients with unresectable metastatic colon cancer is currently greater than 24 months and may be as long as 34 months.68,69 Previous studies have evaluated the role of primary resection in patients with stage IV disease and demonstrated an association with improved survival.70 However, these observational studies are limited by a significant influence of selection bias and outdated chemotherapy regimens. More recent prospective data support only selective primary tumor resection for treatment of symptoms, and patients who are asymptomatic from their primary tumor may therefore be closely followed with serial endoscopic evaluation for obstruction.71 Routine resection of the asymptomatic primary tumor is not recommended.72,73
TUMORRELATED EMERGENCIES
Tumor complications (bleeding, perforation, and obstruction) are serious and potentially lifethreatening conditions of locally advanced tumors. The goals of treatment for these conditions are to 1) avert the immediate negative impact of the complication (eg, death, sepsis), 2) achieve the best possible tumor control, and 3) ensure timely recovery to permit initiation of appropriate adjuvant or systemic treatment.
Bleeding
Surgical resection to stop severe blood loss from localized colon cancer should follow the same oncological principles as in elective resection. Grade of Recommendation: 2C
Acute massive lower GI bleeding from a colon cancer is a rare complication, whereas chronic blood loss is very common. Immediate management includes resuscitation of the patient and potential selective embolization, but surgical resection is the most effective and definitive approach. Preoperative or intraoperative efforts to localize the site of bleeding may be pursued in the clinically stable patient.74 In the very uncommon instance in which the site of bleeding cannot be determined either before operation or intraoperatively, but a colonic tumor is suspected, a subtotal colectomy adhering to oncological principles to each segment of the colon may be considered.
Perforation
Perforation is a lifethreatening complication. After resuscitation of the patient, surgical resection to address both the perforation and the tumor should be performed, if at all possible. Grade of Recommendation: 1B
The overall prognosis of colon perforation due to a colon cancer is significantly worse (associated with advanced tumor disease or sepsis) than perforation from other causes, but it is influenced by factors such as whether the perforation occurs at the tumor proximal to an obstructing tumor in an uninvolved segment of the bowel.75,76 When perforation of uninvolved colon proximal to an obstructing tumor has occurred, whenever possible, resection of the tumor following the oncological principles outlined above should be performed in addition to resection of the perforated segment. In most instances, an ostomy will provide effective fecal diversion and allow for patient recovery until the acute peritonitis has resolved. If the perforation occurs at the site of the tumor but is contained by adjacent structures, resection should ideally incorporate the adjacent structures en bloc. In cases of free perforation with peritonitis, the involved segment should be resected and proximal fecal diversion constructed. A primary anastomosis (with/without proximal diversion) may be considered in selected patients with minimal contamination, healthy tissue quality, and clinical stability.77
Obstruction
The management of patients with an obstructing cancer should be individualized but may include a definitive surgical resection with primary anastomosis. Grade of Recommendation: 1B
Options for the treatment of obstructing tumors depend on the site of obstruction and the presence of proximal colonic distention with fecal load. Options for treatment may include resection with or without anastomosis (eg, Hartmann resection), resection of the distended bowel (eg, subtotal/total colectomy), or temporary relief of obstruction and fecal load (eg, preoperative stenting as a bridge to resection).78,79 The prognosis among patients with obstructing cancers may be worse than among those without obstruction because of the inherently more advanced nature of their disease. However, this does not preclude the potential for curative resection.80
For tumors of the right or transverse colon, a tumordirected resection removes the distended colon segment, and an enterocolonic anastomosis can generally be safely achieved. Performance of an anastomosis in this setting depends on the patient’s general condition at the time of resection and the absence of other factors that indicate the need for a stoma to be created. During curative resection, the principles of oncological resection, including radical lymphadenectomy, should be observed.
A variety of surgical options exists for patients who present with a leftsided colon obstruction from cancer. Appropriate surgical approaches include resection with end colostomy and Hartmann pouch, resection with primary anastomosis, and subtotal colectomy with ileorectal anastomosis. In selected patients, successful preoperative stenting may allow for colonic decompression, metabolic and nutritional recovery, and adequate workup (operability, colonic evaluation) to optimize subsequent elective resection.81 Patients should be carefully selected, however, because a randomized trial of palliative stenting versus surgery was prematurely closed owing to an unexpectedly high rate of perforations in the stented group.82 The selection of the surgical approach should consider the patient’s general condition at the time of resection as well as the quality of the proximal bowel. The morbidity and mortality of a segmental resection, following intraoperative colonic irrigation, among patients with left colonic malignant obstruction has been compared with subtotal colectomy and has not been shown to differ in a randomized trial.83 More recent studies have demonstrated that colonic irrigation may not be mandatory before primary bowel anastomosis in this setting.84,85
Management of Locoregional Recurrence
The treatment of patients with locoregionally recurrent colon cancer should be multidisciplinary, and curative resection should adhere to the principles of primary resection. Grade of Recommendation: 1C
The risk for locoregional recurrence as the first and only site of recurrence following curative resection of localized colon cancer is low, approximately 2% to 3%.86,87 Salvage surgical resection is possible in up to approximately 30% of patients.88 Multimodality treatment, where indicated, provides the patients with the greatest potential for cure with a 5year survival estimate of 27% to 37%.89,90 Factors predictive of prolonged survival following surgical salvage include the completeness of resection (R0), early stage of initial disease, no associated distant disease, unifocal site of recurrence, and lack of retroperitoneal involvement.
Management of Peritoneal Carcinomatosis
The treatment of patients with peritoneal carcinomatosis should be multidisciplinary and individualized and may include surgical cytoreduction. The role of intraperitoneal chemotherapy remains insufficiently defined.
Grade of Recommendation: 2C
Peritoneal carcinomatosis will occur in an estimated 10% to 15% of patients with colorectal cancer. Newer and more effective systemic chemotherapeutic agents and targeted biological therapies have improved survival outcomes in patients with metastatic colorectal cancer. However, there remains a paucity of experimental evidence to guide the treatment of patients with carcinomatosis. The most welldescribed approach includes the combination of cytoreductive surgery in conjunction with perioperative intraperitoneal chemotherapy with or without hyperthermia.91,92 However, most studies report small singlecenter experiences with predominantly 5fluorouracil (5FU)onlybased systemic therapy, and the morbidity associated with cytoreductive surgery with multivisceral resection and hyperthermic intraperitoneal chemotherapy is as high as 60% to 80%.93 As of yet, unanswered questions include the definition of optimal cytoreduction, the impact of systemic therapy by using modern agents, and the role for intraperitoneal chemotherapy.94,95 Registration of patients in clinical treatment protocols is strongly encouraged.
Palliative Procedures
In patients with extensive incurable extent of tumor burden, palliative surgical interventions should be individualized based on the presence of symptoms. Grade of Recommendation: 1B
Patients who present with widely metastatic colon cancer are usually not candidates for surgical cure. Other patients may not be candidates for radical, curative resection because of systemic comorbidities. In these situations, a multidisciplinary approach to potential palliation should be recommended. The goals of palliation should be relief of symptoms caused by the cancer and maintenance of quality of life. In asymptomatic patients, prophylactic resection of the primary tumor is generally not necessary.71,72,96 Patients with asymptomatic primary lesions in the setting of distant metastasis should be referred for systemic chemotherapy unless initial resection of the primary tumor is determined to be the first stage of the multidisciplinary curative treatment plan. Palliative surgical interventions for obstruction of the GI tract or intractable bleeding caused by colon cancer include resection, endoluminal stent therapy, ablative procedures, internal bypass, or creation of a diverting stoma.81 The avoidance of resection in asymptomatic patients allows the patient to more rapidly initiate systemic chemotherapy, averts the risk for surgical morbidity, and results in improved outcomes. While observing patients with intact primary tumors, serial endoscopic evaluation should be performed to detect evidence for progressive disease and permit interventions to avoid acute obstruction. An individual patient’s overall life expectancy should also be considered in the decision for the type of palliative intervention (eg, resection or stent).
Recommendations Regarding Documentation
The surgical report for colorectal cancer should include information regarding the diagnostic workup, intraoperative findings, and technical details of the procedure. Grade of Recommendation: 1C
The ideal surgical report should clearly communicate the workup, intraoperative findings, and technical details of the procedure. The report should include a description of preoperative treatments and relevant workup and findings on exploration, including the presence of synchronous metastases or gross involvement of mesenteric lymph nodes, tumor site, and adjacent organ involvement. The report should also describe treatment details, including type of incision, extent of bowel and mesenteric resection, anastomotic technique, en bloc resection of contiguously involved organs, and an intraoperative assessment of the completeness of resection including margin status.14,21,28
Adjuvant Therapy
Decisions regarding adjuvant treatment following curatively resected colon cancer should be based on the clinical findings at resection, including stage of disease and patient comorbidities. The choice of the adjuvant chemotherapy regimen should be made jointly by the patient and the physician. Radiation therapy plays a minimal role in the adjuvant treatment of colon cancer.
Adjuvant chemotherapy should be recommended for patients with stage III colon cancer. Grade of Recommendation: 1A
A number of large multiinstitutional US and international randomized clinical trials have demonstrated the survival benefit with adjuvant chemotherapy. Pooled data from randomized trials demonstrates a 30% reduction in the risk for recurrence and a 26% reduction in the risk for death with fluoropyrimidinebased therapy administered for 6 months.97–99 More recently, the addition of oxaliplatin to fluoropyrimidine (eg, 5FU) chemotherapy has been shown to effect an additional approximately 20% reduction in relative risk for recurrence or death corresponding to an approximately 5% absolute survival benefit at 5 years with combination 5FU and leucovorin (LV) with oxaliplatin in comparison with 5FU alone.100,101 Therefore, the firstline adjuvant chemotherapy regimen should include a fluoropyrimidine (5FU/LV or capecitabine) and oxaliplatin. However, grade 3 peripheral sensory neuropathy occurs in approximately 12% of patients who receive oxaliplatin, which may make it unsuitable for some patients.102 The addition of irinotecan in combination with 5FU was studied in several phase III randomized controlled trials in the United States and internationally and was demonstrated to yield no survival benefit when compared to 5FU/LV alone.103,104 At present, there is no role for the addition of irinotecan in the adjuvant setting after resection of localized colon cancer.
The role of the biological agents, such as the vascular endothelial growth factor inhibitor bevacizumab or the epidermal growth factor receptor inhibitor cetuximab and panitumumab along with other targeted agents, has been the subject of recent investigation. Unfortunately, 3 separate phase III multiinstitutional trials have failed to demonstrate added benefit with the addition of either bevacizumab (NSABP C08, AVANT) or epidermal growth factor receptor inhibitors (NCCTG N0147) to FOLFOX alone105–107 At present, there is no evidence to support the routine addition of biological agents in the adjuvant setting.
Adjuvant chemotherapy may be considered for patients with highrisk stage II colon cancer. Grade of Recommendation: 2B
There are conflicting data regarding the role of adjuvant chemotherapy in stage II colon cancer. Most of the previous randomized trials of adjuvant therapy for colon cancer have enrolled both stage II and stage III patients, and some have demonstrated a small difference corresponding to a potential absolute improvement in overall survival of approximately 2% to 3% with 5FU/LV and 3% to 4% with FOLFOX.101,108–110 However, the proportion of patients with stage II cancers were approximately 20% to 25% in these trials, and definitive conclusions, given such a small effect, have not been possible. However, a subgroup of highrisk patients with nodenegative colon cancer may benefit. In the United Kingdom, a study of 5FU adjuvant chemotherapy for stage II colon cancer was associated with a 20% relative risk reduction for recurrence associated with adjuvant therapy. However, a significant proportion of the patients had fewer than 12 lymph nodes examined, and the potential impact of understaging in this study is unknown.111 The benefit of adjuvant chemotherapy in stage II patients has not been definitively shown, and patients with stage II colon cancer should be encouraged to participate in adjuvant therapy clinical trials. Patients with stage II colon cancer are considered to be at high risk in the presence of T4 stage, perforation, peritumoral lymphovascular or neural involvement, or poorly differentiated histology. In addition, those patients in whom fewer than 12 lymph nodes were evaluated may also be considered to be at high risk, and adjuvant chemotherapy may be recommended.3
More recently, new molecular prognostic markers have emerged and are under investigation. Tumors that are microsatellite instabilityhigh (MSIH) appear to have improved prognosis and limited benefit from 5FUbased chemotherapy. Conversely, loss of heterozygosity at chromosome 18q (DCC) has been associated with poor prognosis, although the true value of this factor has not yet been fully elucidated.4,112 Although recently developed and commercially available genomic profiling tools have demonstrated prognostic information in patients with stage II colon cancers, their utility for determining treatment response could not be established, and there is no clear role for their use in treatment stratification.113,114
