Practice Parameters for the Management of Rectal Cancer (Revised)
Prepared by
The Standards Practice Task Force
The American Society of Colon and Rectal Surgeons
Joe J. Tjandra, M.D., John W. Kilkenny, M.D., W. Donald Buie, M.D.,
Neil Hyman, M.D., Clifford Simmang, M.D., Thomas Anthony, M.D.,
Charles Orsay, M.D., James Church, M.D., Daniel Otchy, M.D., Jeffrey Cohen, M.D.,
Ronald Place, M.D., Frederick Denstman, M.D., Jan Rakinic, M.D.,
Richard Moore, M.D., Mark Whiteford, M.D.
The American Society of Colon and Rectal Surgeons is dedicated to assuring highquality patient care by advancing the science, prevention, and management of disorders and diseases of the colon, rectum, and anus. The Standards Committee is composed of Society members who are chosen because they have demonstrated expertise in the specialty of colon and rectal surgery. This Committee was created to lead international efforts in defining quality care for conditions related to the colon, rectum, and anus. This is accompanied by developing Clinical Practice Guidelines based on the best available evidence. These guidelines are inclusive, and not prescriptive. Their purpose is to provide information on which decisions can be made, rather than dictate a specific form of treatment. These guidelines are intended for the use of all practitioners, health care workers, and patients who desire information about the management of the conditions addressed by the topics covered in these guidelines. It should be recognized that these guidelines should not be deemed inclusive of all proper methods of care or exclusive of methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding the propriety of any specific procedure must be made by the physician in light of all of the circumstances presented by the individual patient.
STATEMENT OF THE PROBLEM
Colorectal adenocarcinoma is the second leading cause of cancer deaths in western countries. Rectal cancer comprises approximately 25 percent of the malignancies arising in the large bowel. The estimated occurrence of new rectal cancer cases in the United States was projected to be 40,570 during 2004.1
Anatomically, the rectum is the distal 18cm of the large bowel leading to the anal canal.2 Cancers of the intraperitoneal rectum behave like colon cancers with regard to recurrence patterns and prognosis.3 By contrast, the extraperitoneal rectum resides within the confines of the bony pelvis; it is this distal 10 to 12 cm that constitutes the rectum from the oncologic standpoint.
PREOPERATIVE ASSESSMENT
1. Patients should be evaluated for their medical fitness to undergo surgery. When an ostomy is a consideration, preoperative counseling with an enterostomal therapist should be offered when available. Level of Evidence: III; Grade of Recommendation: B.
Appraisal of operative risk, especially with respect to cardiopulmonary comorbidity, is an essential part of the preoperative process. History and physical examination are the cornerstones of diagnostic evaluation and may prompt further investigation and intervention to optimize operative risk. In selected cases, a nonsurgical approach to the lesion may be necessary. Several perioperative, riskassessment scoring systems have been published to help guide the surgeon.4–6 The need for ancillary laboratory tests is guided by history and physical examination.
Retrospective studies have indicated that patients who had access to enterostomal therapy counseling before surgery enjoyed a better quality of life postoperatively.7 Thus preoperative siting and counseling by an enterostomal therapist helps to improve outcomes in patients requiring a stoma.8
2. Clinical assessment should include a family history to identify patients with familial cancer syndromes and to evaluate familial risk. Level of Evidence: III; Grade of Recommendation: B.
A family medical history should be taken from patients with rectal cancer to identify close relatives with a cancer diagnosis. The clinician should look for patterns consistent with the genetic syndromes of hereditary nonpolyposis colorectal cancer, familial adenomatous polyposis, and familial colorectal cancer because this may affect surgical decisions.9
The colorectal cancer risk in family members increases with the number of affected members, the closeness of the relationship to the patient, and earlier age of onset.10,11 Medical information that patients provide about their relatives often is inaccurate.12–16 If a family medical history seems to be significant but proves difficult to confirm, it may be appropriate to seek expert help from a familial cancer clinic.
3. Digital rectal examination and rigid proctosigmoidoscopy are typically required for accurate tumor assessment. Level of Evidence: Class V; Grade of Recommendation: D.
Digital rectal examination enables detection and assessment of the size and degree of fixation of mid and low rectal tumors. Although digital assessment of the extent of local disease may be imprecise, it provides a rough estimate of the local staging of rectal cancer.17 Rigid proctosigmoidoscopy is usually performed in conjunction with the digital rectal examination. It usually allows the most precise assessment of tumor location and the distance of the lesions from the anal verge. These issues are critical in optimizing preoperative planning.
4. Full colonoscopy should be performed to exclude synchronous neoplasms. Barium enema may be used for those patients unable to undergo complete colonoscopy. Level of Evidence: III; Grade of Recommendation: B.
Colonoscopy is currently the most accurate tool for screening the colon and rectum for neoplasms.18 The sensitivity of colonoscopy for colon cancer is typically in the range of 95 percent.19–21 Colonoscopy allows biopsy and histologic confirmation of the diagnosis. It also allows for identification and endoscopic removal of synchronous polyps. A study by the U.S. National Polyp Study found that colonoscopy was significantly more accurate than doublecontrast barium enema in diagnosing colorectal polyps.18
5. CT scanning of the abdomen and pelvis and transrectal ultrasound (TRUS) or magnetic resonance imaging (MRI) should typically be performed in patients who are potentially surgical candidates. Level of Evidence: III; Grade of Recommendation: B.
Transrectal ultrasound has emerged as the diagnostic modality of choice for preoperative local staging of mid and distal rectal cancers.22 Abdominal and pelvic CT scans often provide highly useful information regarding the presence of distant metastases as well as adjacent organ invasion in advanced lesions. However, its role in local staging is limited.23,24 TRUS more accurately assesses bowel wall penetration and lymph node involvement.25 MRI, bolstered by the recent introduction of phased array coils, has improved spatial resolution. Overall MRI has similar accuracy to TRUS in tumor staging. MRI seems to be more accurate in assessing T3 and T4 lesions, whereas TRUS may be more accurate in defining earlierstage lesions (T1, T2).26,27 Nodal staging seems to be comparable between TRUS and MRI. MRI has the added advantage of a multiplanar and larger field of view of the mesorectal fascia and more accurately predicts the likelihood of obtaining a tumorfree circumferential resection margin.28,29 Because of technical reasons, TRUS is less useful for the evaluation of more proximal rectal cancers. Both modalities have interobserver issues and a demonstrable learning curve. TRUS is more accessible, portable, and less expensive.
6. Routine chest radiographs or chest CT scanning should usually be performed. Level of Evidence: III; Grade of Recommendation: B.
Rectal cancer is more likely than colon cancer to be associated with lung metastases without liver metastases. The finding of pulmonary metastases often will alter patient management decisions and therefore is warranted in most clinical situations. Abnormal findings on plain radiographs usually warrant chest CT scanning.30
7. Carcinoembryonic antigen level should usually be determined preoperatively. Level of Evidence: III; Grade of Recommendation: B.
Carcinoembryonic antigen (CEA) level is most useful when found to be elevated preoperatively and then normalizes after resection of the tumor. Subsequent elevations suggest recurrence or metastatic disease. Because of a lack of sensitivity and specificity, its utility as a screening test has never been demonstrated.31 Preoperative liver function tests may suggest metastatic disease, but are nonspecific and insensitive. Therefore, routine liver function tests are not warranted.32
TREATMENT CONSIDERATIONS
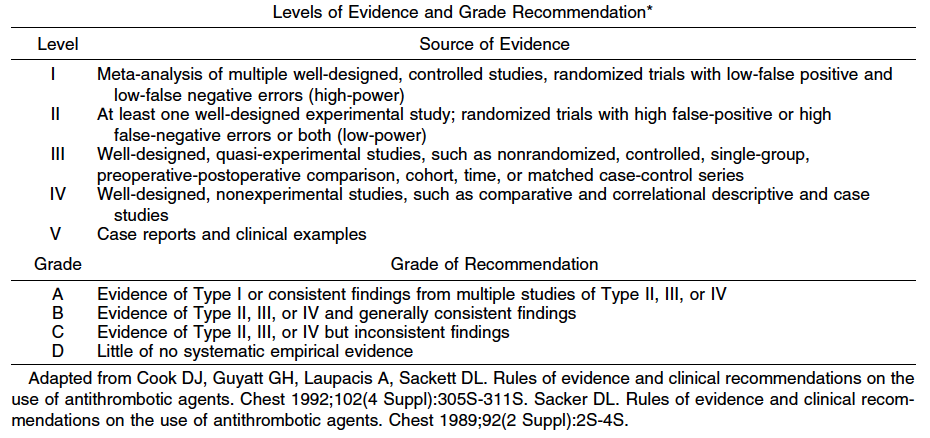
Surgery is the mainstay of treatment for rectal cancer. The risk of recurrence is dependent on the TNM stage (Table 1).33 Early stage cancer can be treated by surgical resection alone. More advanced lesions require adjuvant therapy to increase the probability of cure.34
The surgeon is a critical variable with respect to morbidity, sphincter preservation rate, and local recurrence.35–38 Phillips found that local recurrence ranged from <5 to 15 percent amongst different surgeons with no difference in case mix.39 In a Scottish study,40 the operative mortality and tenyear survival rate after “curative” surgery varied with the surgeon, ranging from 0 to 20 percent and 20 to 63 percent, respectively. Adequate training35,41 and surgical volume35,42,43 both seem to be important factors. These data emphasize the technical aspect of rectal cancer surgery and the need for a standardized surgical approach.
SURGICAL THERAPY
Resection Margin
A 2cm distal margin is adequate for most rectal cancers. Level of Evidence: Class III; Grade of Recommendation: B.
In smaller cancers of the low rectum without adverse histologic features, a 1cm distal margin is acceptable. Level of Evidence: Class III; Grade of Recommendation: B.
The principle objective of surgical treatment is to obtain clear surgical margins.44 The proximal resection margin is determined by blood supply considerations. Multiple studies have demonstrated that 81 to 95 percent of rectal cancers have intramural spread <1 cm from the primary lesion.45–49 Rectal carcinomas with intramural spread beyond 1 cm tend to be highgrade, nodepositive, or have distant metastases45–48 In the majority of cases, a distal surgical margin of 2 cm would remove all microscopic disease. In patients with advanced disease, more extensive microscopic intramural disease may be present, but the resection is typically palliative because of a high likelihood of occult distant metastases.46,50 For cancers of the distal rectum (<5 cm from the anal verge), the minimum acceptable length of the distal margin is 1 cm.51–54 Margins >1 cm should be obtained with larger tumors, especially those demonstrating adverse histologic features.55 The margins of resection should be measured in the fresh, pinned out specimen. The formalinfixed specimen may shrink up to 50 percent in length.45
Level of Proximal Vascular Ligation
Proximal lymphovascular ligation at the origin of the superior rectal artery is adequate for most rectal cancers. Level of Evidence: Class III; Grade of Recommendation: B.
Appropriate lymphadenectomy is based on the ligation of the major vascular trunks. There is no demonstrable survival advantage for a high ligation of the inferior mesenteric artery at its origin. Available evidence suggests that for colorectal cancer without clinically suspicious nodal disease, removal of lymphovascular vessels up to the origin of the primary feeding vessel is adequate.56–58 Thus for rectal cancer, this is at the origin of the superior rectal artery, just distal to the origin of the left colic artery.59 In patients with lymph nodes thought to be involved clinically, removal of all suspicious nodal disease up to the origin of inferior mesenteric artery is recommended.57 Suspicious periaortic nodes may be biopsied for staging purposes. High ligation of the inferior mesenteric vessels may be helpful to provide additional mobility of the left colon, as often is required for a low colorectal anastomosis or a colonic Jpouch construction.60
Circumferential Resection Margin
For distal rectal cancers, total mesorectal excision (TME) is recommended. For upper rectal cancers, a tumorspecific mesorectal resection is adequate. Level of Evidence: Class II; Grade of Recommendation: A.
The mesorectum is the fatty tissue that encompasses the rectum. It contains lymphovascular and neural elements. Surgical excision of the mesorectum is accomplished by sharp dissection in the plane between the fascia propria of the rectum and the presacral fascia. Radial clearance of mesorectal tissue enables the en bloc removal of the primary rectal cancer with any associated lymphatic, vascular, or perineural tumor deposits. Total mesorectal excision is associated with the lowest reported local recurrence rates.61–63
The importance of en bloc resection of an intact mesorectum is supported by pathologic studies that demonstrated tumor deposits in the mesorectum separate from the primary tumor.64,65 A similar local recurrence rate has been noted by others who practice wide anatomic resection in the mesorectal plane without routine total mesorectal excision.66,67 The degree of mesorectal involvement on pathologic examination correlates with recurrence and survival.65 Pathologic assessment of rectal cancer specimens suggests that distal mesorectal spread may occur up to 4 cm away from the primary tumor.68,69 Thus, a cancer in the distal rectum should be treated with a total mesorectal excision in most cases.70 Upper rectal cancers may be treated with a tumorspecific mesorectal resection.
Pathologic studies also have drawn attention to the circumferential margin and the importance of radial clearance. In a prospective study by Quirke et al.,71 when the resected specimen had negative lateral margins, cancer recurred locally in only 3 percent of cases compared with an 85 percent local recurrence rate if the lateral margins were involved with tumor. Pathologic studies of mesorectal specimens have confirmed these findings.72–75 In the presence of negative circumferential margins, specimens with an intact or nearly intact mesorectum are associated with a lower overall recurrence rate compared with an incomplete specimen.75
Circumferential margin involvement in the presence of an intact mesorectal specimen is a strong predictor for local recurrence and is independent of TNM classification. This finding is a marker for advanced or aggressive disease rather than inadequate surgery.65,72,76,77 In a large, randomized study, a margin of 2 mm between tumor and the mesorectal fascia was considered positive and was associated with a higher local recurrence rate (16 vs. 5.8 percent; P < 0.0001).75 Furthermore, patients who had a margin 1 mm had an increased risk of distant metastases (37.6 vs. 12.7 percent; P < 0.0001). Finally, support for the importance of mesorectal excision also comes from a surgical teaching initiative in the county of Stockholm. The widespread adoption of mesorectal excision for mid and low rectal cancers significantly reduced the local recurrence rate by >50 percent and improved rectal cancer mortality.78 These results along with the recent Dutch trial are evidence that a standardized surgical approach can reduce the variability of surgical outcomes.79
There is inadequate evidence to support a routine extended lateral lymphadenectomy in addition to mesorectal excision. Clinically suspicious nodal disease in the lateral pelvic sidewall should be removed if technically feasible or biopsied for staging purposes.80
En Bloc Resection of Adherent (T4) Tumors
Rectal cancers with adjacent organ involvement should be treated by en bloc resection. Level of Evidence: Class III; Grade of Recommendation: B.
Tumors may be adherent to adjacent organs by malignant invasion or inflammatory adhesions.81,82 Locally invasive rectal cancer (T4) is removed by an en bloc resection to include any adherent tissues. If a tumor is transected at the site of local adherence, resection is deemed incomplete, because it is associated with a higher incidence of treatment failure.82 An en bloc resection with clear margins including adjacent organs involved by local invasion can achieve survival rates similar to those of patients with tumors that do not invade an adjacent organ.81,83–85
Inadvertent Perforation
Inadvertent perforation of the rectum worsens oncologic outcome and should be documented. Level of Evidence: Class III; Grade of Recommendation: B.
Inadvertent rectal perforation during the resection of rectal cancer is associated with a statistically significant reduction in fiveyear survival and an increase in local recurrence rates.86–88 Perforation at the site of the cancer has an even greater adverse impact on local recurrence and survival than a perforation remote from the tumor site.88 Inadvertent perforation of the rectum and resultant intraoperative spillage of tumor cells should be documented and considered in postoperative adjuvant treatment decisions and outcome measurements.
Other Operative Considerations
1. Grossly normal ovaries need not be removed. Level of Evidence: Class III; Grade of Recommendation: B.
Ovarian metastases from rectal cancer occur in up to 6 percent of patients and are usually associated with widespread disease and poor prognosis.89 There are no data to support routine prophylactic oophorectomy.90,91 Direct invasion of the ovary is treated with an en bloc resection. Oophorectomy should be considered if the organ is grossly abnormal in postmenopausal females or in females who have received preoperative pelvic radiotherapy. Bilateral oophorectomy is indicated if only one ovary is involved, because there is a high risk of occult metastatic disease in the contralateral ovary.92
2. There is insufficient evidence to recommend intraoperative rectal washout. Level of Evidence: Class IV; Grade of Recommendation: C.
Viable exfoliated malignant cells have been demonstrated in the bowel lumen of patients with primary rectal cancer.93–95 Intraoperative rectal washout, before an anastomosis, is performed by many surgeons with the intention of reducing locoregional recurrence. There is insufficient evidence to recommend this practice.
3. Curative local excision is an appropriate treatment modality for carefully selected T1 rectal cancers. Level of Evidence: Class II; Grade of Recommendation: B.
Local excision of rectal cancer is an appropriate alternative therapy for selected cases of rectal cancer with a low likelihood of nodal metastases. This probability is dependent on the depth of tumor invasion (T stage), tumor differentiation and lymphovascular invasion.96–98 Comparative trials to abdominoperineal resection support transanal local excision with curative intent for T1, welldifferentiated cancers that are <3 cm in diameter and occupy <40 percent of the circumference of the rectal wall.97,99,100 The depth of mural penetration is correlated with the risk of nodal metastases. For tumors confined to the submucosa, associated nodal metastases have been seen in 6 to 11 percent of patients; for cancer invading the muscularis propria, there was a 10 to 20 percent risk of nodal metastases, and with tumors extending into the perirectal fat, this risk increased to 33 to 58 percent.101 Brodsky and colleagues96 examined 154 specimens and found a 12 and 22 percent incidence of lymph node metastases in T1 and T2 tumors respectively. In addition, the incidence of lymph node metastases increases dramatically with increasing tumor grade; lymph nodes are positive in up to 50 percent of poorly differentiated tumors.96 The tumor must be excised intact by fullthickness excision with clear margins. It should be orientated and pinned out for complete pathologic examination. If unfavorable features are observed on pathologic examination, a radical excision is warranted.97,102 Transanal endoscopic microsurgery uses similar surgical principles as a transanal local excision, but is designed to remove lesions up to approximately 20 cm from the anal verge.97,103,104 Both transanal local excision and transanal endoscopic microsurgery may afford reasonable palliation for patients with metastatic disease who are poor candidates for a more extensive surgical procedure. 4. Laparoscopicassisted resection of rectal cancer is feasible but requires specific surgical expertise. Its oncologic effectiveness remains uncertain at this time. Level of Evidence: Class II; Grade of Recommendation: B. Laparoscopic techniques for rectal resection are established and feasible.105,106 In two randomized studies on colon cancer, laparoscopicassisted colon resection had similar recurrence rates to conventional open resection107,108; however, the oncologic effectiveness of laparoscopic surgery for the curative treatment of rectal cancer is not yet fully resolved. A single, randomized study suggests that laparoscopicassisted resection for rectosigmoid cancer is safe and effective.109 The major hindrance to a wide adoption of laparoscopicassisted resection is the steep learning curve. Technically, a restorative anastomosis for mid rectal cancer may be difficult to perform laparoscopically. Handassisted laparoscopic techniques may expand the indications for laparoscopic resections; however, there is inadequate evidence at this time to support this claim.110 5. Emergency intervention: Primary resection of an obstructing or perforated carcinoma is recommended unless medically contraindicated. Level of Evidence: Class III; Grade of Recommendation: A. Hemorrhage, obstruction, and bowel perforation are the most common indications for emergency intervention for rectal cancer. Appropriate management must be individualized with options, including resection with anastomosis and proximal diversion, or diversion alone followed by radiation. Other alternatives include endoluminal stenting or laser/cautery recanalization. Selfexpandable metallic stents can be used to relieve obstruction by a proximal rectal cancer. This allows for mechanical bowel preparation, elective resection, and anastomosis. In some cases with advanced metastatic disease or major comorbidities, it may constitute definitive treatment. Stents are successfully deployed in 80 to 100 percent of cases.111 Complications include perforation (5 percent), stent migration (10 percent), bleeding (5 percent), pain (5 percent), and reobstruction (10 percent). In the setting of a perforated rectal cancer, the treatment of choice is resection, copious peritoneal washout, pelvic drainage, and construction of a sigmoid end colostomy.112,113 ADJUVANT THERAPY 1. Adjuvant chemoradiation should be offered to patients with Stage II and III rectal cancers. Level of Evidence: Class I; Grade of Recommendation: A. Adjuvant or neoadjuvant chemotherapy and pelvic radiation should be offered to patients with Stage II and III rectal cancers. These patients have been shown in multiple trials to have a higher risk of local and distant relapse if surgery alone is performed. Improved cancerspecific survival has been reported with both preoperative and postoperative adjuvant treatment. Postoperative adjuvant therapy has been the standard for locally advanced resectable rectal cancer. Initial trials examined postoperative radiotherapy alone as an adjunct to surgical resection. The Colorectal Cancer Collaborative Group metaanalysis of trials comparing surgery and postoperative radiation vs. surgery alone showed that postoperative radiotherapy significantly reduced local recurrence by approximately onethird (odds ratio (OR), 0.73; 95 percent confidence interval (CI), 0.55–0.96); however, overall survival was unaffected.114 A second metaanalysis analyzed eight trials and reported similar findings.115 The use of postoperative chemotherapy alone also has been investigated in several randomized, controlled trials. GITSG 7175 compared postoperative adjuvant chemotherapy alone to observation in resectable rectal cancer.116 There was a nonsignificant trend toward improved cancerfree survival with chemotherapy. The NSABP R01 trial compared chemotherapy to surgery alone or radiation therapy alone in 555 patients. A significant overall improvement in diseasefree and overall survival was found with the use of chemotherapy.117 When these two trials were pooled with a Japanese trial118 in a meta analysis, a significant improvement in survival for chemotherapy was observed (OR, 0.65; 95 percent CI, 0.51–0.83; P = 0.0006)119; however, no difference in local recurrence was observed (OR, 0.71; 95 percent CI, 0.41–1.16; P = 0.17). In a second metaanalysis of 4,960 patients with colorectal cancer from three randomized trials or comparing adjuvant chemotherapy with oral fluoropyrimidines (5fluorouracil (5FU), tegafur, or carmofur) to surgery alone, subgroup analysis of 2,310 patients with rectal cancer demonstrated an improvement in mortality (relative risk (RR), 0.857; 95 percent CI, 0.73–0.999; P = 0.049) and diseasefree survival (RR, 0.767; 95 percent CI, 0.656–0.882; P = 0.00003) for patients receiving adjuvant oral chemotherapy.120 Finally, a metaanalysis by Sakamoto and colleagues121 of three trials comparing postoperative oral carmofur with surgery alone demonstrated a highly significant effect for the subgroup of Dukes C rectal cancer treated with adjuvant oral chemotherapy in both diseasefree and overall survival. The NSABP R02 trial randomized 694 Stage II and III patients to receive postoperative chemotherapy (MOF or 5FULV) alone or postoperative chemotherapy with radiotherapy. Although the addition of radiotherapy conferred no advantage in diseasefree or overall survival, it reduced the cumulative incidence of local regional relapse (8 vs. 13 percent; P = 0.02).122 Because chemotherapy alone does not seem to reduce local recurrence, the use of chemotherapy alone is not standard practice in the treatment of rectal cancer. Two randomized, controlled trials have compared combined modality therapy (CMT) for Stage II and III rectal cancer to surgery alone.116,123 The local recurrence rates for the surgeryalone arm were 25 percent116 and 30 percent123 respectively. In both of these studies, postoperative CMT significantly reduced the local recurrence rate and improved overall survival. Krook et al.124 randomized 204 patients with highrisk rectal cancer to postoperative radiotherapy alone or CMT. The CMT arm experienced lower recurrence rates, both locally and distantly. The rates of cancerrelated deaths and deaths from any cause were also significantly reduced with CMT. The morbidity associated with postoperative adjuvant therapy can be significant.125 In the Danish,126 Dutch,127 and MRC128 postoperative therapy trials, 20 percent of patients did not complete their allocated treatment because of postoperative complications and/or patient refusal. Furthermore, functional outcomes may be compromised by postoperative CMT. In a review of two NSABP trials, a significant increase in severe diarrhea was noted from CMT particularly in patients receiving a low anterior resection.129,130 Other acute side effects included cystitis, skin reactions, and fatigue. Ooi et al.125 emphasized both acute and chronic effects, including radiation enteritis, smallbowel obstruction, and rectal stricture. Preoperative or neoadjuvant therapy is an attractive alternative to postoperative adjuvant therapy and offers a number of theoretic and practical advantages. It can be given as short course (2,500 cGy during 5 days) or as long course (5,040 cGy during 42 days) with chemotherapy. There are three metaanalyses comparing preoperative radiotherapy to surgery alone in resectable rectal cancer.114,131,132 Two analyses found a significant reduction in overall mortality.131,132 When all three analyses were pooled, preoperative radiation decreased the local recurrence rate by approximately 50 percent and increased survival by 15 percent compared with surgery alone. The absolute reduction in local recurrence was 8.6 percent (95 percent CI, 3.1–14.2 percent) with an absolute reduction in fiveyear mortality of 3.5 percent (95 percent CI, 1.1–6 percent).132 Although preoperative radiation alone has a significant effect on local recurrence, it is not as effective as postoperative chemoradiotherapy in improving survival. Thus, if shortcourse preoperative radiotherapy is used, chemotherapy should be added postoperatively, at least in Stage III disease.132 Many of the trials included for analysis reported local recurrence rates in the “surgery only” groups that far exceed what has been reported with total mesorectal excision. The question has been raised whether adjuvant therapy is required in patients who have undergone “optimal” surgery. In a recent randomized trial, total mesorectal excision was performed with or without a fiveday regimen of preoperative shortcourse radiotherapy.133 The twoyear local recurrence rate was improved by the use of preoperative radiotherapy (2.4 vs. 8.2 percent respectively), indicating that preoperative radiation therapy reduces local recurrence rates even after “optimal” surgery. However, there was no significant difference in the overall survival rates after a median followup period of two years. Preoperative radiotherapy did not benefit the subset of patients in whom the circumferential resection margin was positive. More mature followup data is awaited, but there is unlikely to be any improvement in survival, given the small benefit in local recurrence rate. A single, randomized study compared conventional shortcourse preoperative RT with selective postoperative RT for Stage II and III patients. The local recurrence rate was significantly lower after preoperative RT (11 vs. 22 percent respectively).134 Morbidity rates were lower for the preoperative group; however, this may be because of the higher postoperative radiation dose given to the highrisk patients.135 Several trials are maturing that compare preoperative and postoperative chemoradiation. The CAO/ARO/AIO94 trial compared preoperative and postoperative CMT with > 800 patients accrued. Early results have found no difference in postoperative complications or acute toxicities between the groups; however, a higher sphincter preservation rate was reported for the preoperative group.136 A recent update has shown a significant reduction in local recurrence with preoperative therapy.137 In addition, there was less stenosis at the anastomotic site and better sphincter preservation in lowlying tumors after preoperative therapy. The Polish Colorectal Study Group trial has recently completed accrual comparing conventional longcourse 50.4 Gy radiotherapy combined with bolus 5FU/LV to shortcourse radiotherapy (25 Gy in 5 days) before total mesorectal excision.138 Early data indicates that the longcourse CMT arm was associated with greater frequency and severity of acute toxicity. CMT caused greater tumor shrinkage, but there was no difference in sphincter preservation rate. The NSABP R03 trial also compared preoperative vs. postoperative CMT.139,140 The chemotherapy protocol involved a potential delay of surgery for up to seven months. There was evidence of local downstaging with a complete tumor pathologic response in 8 percent of the patients undergoing preoperative CMT. Early results of this trial again suggested again that a larger proportion of the preoperative patients had sphinctersparing surgery, but suffered higher toxicity from the treatment. More mature data will be forthcoming from these three trials.
A major concern of shortcourse RT remains the increase in shortterm and longterm toxicity, as has been noted with shortcourse RT at other sites.141 A subgroup of patients from the Swedish Rectal Cancer Trial completed a questionnaire regarding anorectal dysfunction.142 Abnormal function included frequency, urgency and incontinence, and reduced social activities in 30 percent of patients who received shortcourse radiation vs. 10 percent of patients after surgery alone (P < 0.01). The authors suggested a radiation effect on the anal sphincter or its nerve supply.143 These complications are similar to those after postoperative radiotherapy.
The practice parameters set forth in this document have been developed from sources believed to be reliable. The American Society of Colon and Rectal Surgeons makes no warranty, guarantee, or representation whatsoever as to the absolute validity or sufficiency of any parameter included in this document, and the Society assumes no responsibility for the use or misuse of the material contained.
