Practice Parameters for the Management of Rectal Prolapse
Madhulika Varma, M.D. Janice Rafferty, M.D. W. Donald Buie, M.D.
Prepared by the Standards Practice Task Force of the American Society of Colon and Rectal Surgeons
The American Society of Colon and Rectal Surgeons is dedicated to ensuring high-quality patient care by advancing the science, prevention, and management of disorders and diseases of the colon, rectum, and anus. The Standards Committee is composed of Society members who are chosen because they have demonstrated expertise in the specialty of colon and rectal surgery. This Committee was created to lead international efforts in defining quality care for conditions related to the colon, rec-tum, and anus. This is accompanied by developing Clinical Practice Guidelines based on the best available evidence. These guidelines are inclusive, and not prescriptive. Their purpose is to provide information on which decisions can be made, rather than dictate a specific form of treatment. These guidelines are intended for the use of all practitioners, health care workers, and patients who desire information about the management of the conditions addressed by the topics covered in these guidelines.
It should be recognized that these guidelines should not be deemed inclusive of all proper methods of care or exclusive of methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding the propriety of any specific procedure must be made by the physician in light of all of the circumstances presented by the individual patient.
STATEMENT OF THE PROBLEM
Rectal prolapse, internal intussusception, and solitary rectal ulcer syndrome comprise a spectrum of anatomical ab-normalities involving descent of full- or partial-thickness rectal wall associated with pelvic floor dysfunction. These conditions, although benign, can be extremely debilitating because of the discomfort of prolapsing tissue both internally and externally, associated drainage of mucus or blood, and the common occurrence of fecal incontinence or constipation. In patients with rectal prolapse, diastasis of the levator ani, an abnormally deep cul-de-sac, a redundant sigmoid colon, a patulous anal sphincter, and loss of the rectal sacral attachments are commonly found.1– 6 In times past, restoration of normal anatomy to treat rectal prolapse was considered a definition of success. However, the presence of multiple operations to correct this problem indicates that the achievement of excellent outcomes is somewhat elusive.
Women aged 50 and older are 6 times as likely as men to present with rectal prolapse.7–9 Although it is commonly thought that rectal prolapse is a consequence of multi-parity, approximately one-third of female patients with rectal prolapse are nulliparous. The peak age of incidence is the seventh decade in women, whereas the relatively few men who have this problem may develop pro-lapse at the age of 40 or less. One striking characteristic of younger patients is their increased tendency to have autism, syndromes associated with developmental delay, and psychiatric comorbidities requiring multiple medica-tions.10 Young male patients with rectal prolapse also tend to report significant symptoms related to bowel function, specifically evacuation.
Approximately 50% to 75% of patients with rectal prolapse report fecal incontinence, and 25% to 50% of patients will report constipation.11–15 Incontinence in the setting of rectal prolapse may be explained by the presence of a direct conduit (the prolapse) bypassing the sphincter mechanism, the chronic stretch and trauma to the sphincter caused by the prolapse itself, and continuous stimulation of the rectoanal inhibitory reflex by the prolapsing tissue. Pudendal neuropathy has been demonstrated in up to one-half of patients with prolapse16 and maybe responsible for denervation-related atrophy of the external sphincter musculature.17 Constipation associated with prolapse may result from intussuscepting bowel in the rec-tum creating a blockage that is exacerbated with straining, pelvic floor dyssynergia, and colonic dysmotility.13,14
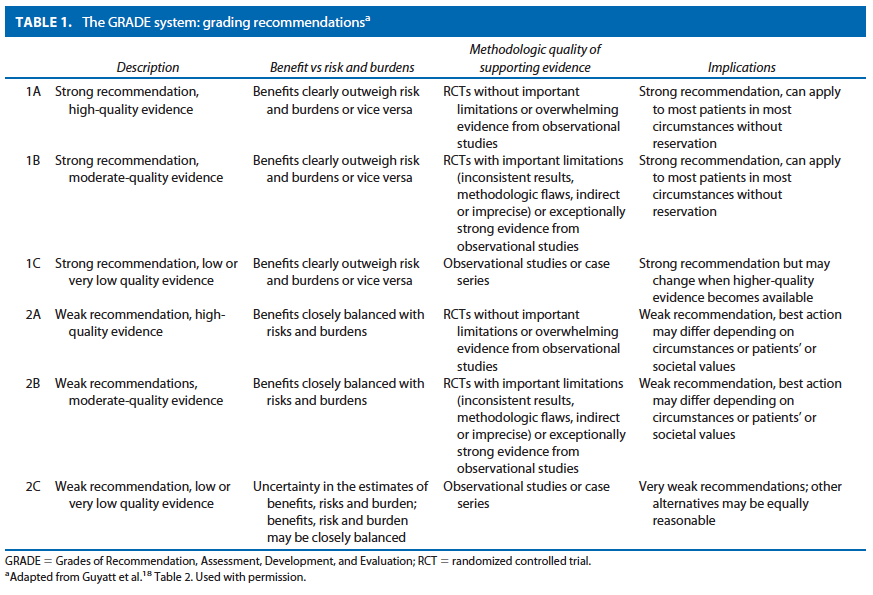
METHODOLOGY
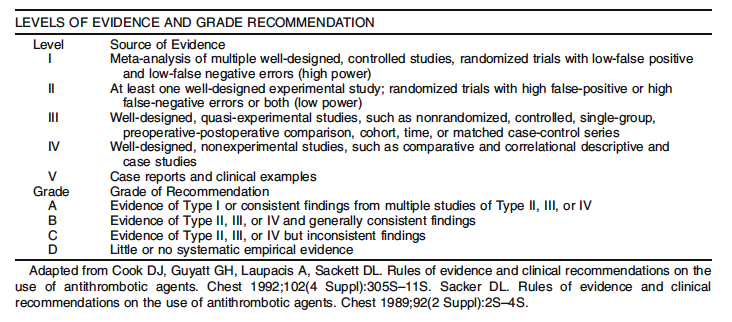
An organized search of MEDLINE/PubMed and the Cochrane Database of Systematic Reviews and Clinical Trials was performed, from 1978 to June 2010, using the key words “rectal prolapse,” “procidentia,” “laparoscopy,” “suture rectopexy,” “mesh rectopexy, resection rectopexy,” “perineal rectosigmoidectomy.” Selected embedded references were also reviewed. All English language manuscripts and studies of adults were reviewed. Recommendations were formulated by the primary authors and reviewed by the entire committee. The final grade of recommendation was performed using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) system18 (Table 1) and reviewed by the entire Standards Committee.
RECOMMENDATIONS
Evaluation of Rectal Prolapse
1. The initial evaluation of a patient with rectal prolapse should include a complete history and physical examination. Grade of Recommendation: Strong recommendation based on low quality evidence 1C
Before operative intervention, a careful history and physical examination should be performed. If the diagnosis is suspected from the history, but not detected on physical examination, confirmation can be obtained by asking the patient to reproduce the prolapse by straining while on a toilet with or without use of an enema. Inspection of the perineum with the patient in the sitting or squatting position is helpful for this purpose. A common pitfall in the diagnosis of rectal prolapse is the potential for confusion with prolapsing internal hemorrhoids or rectal mucosal prolapse. Usually, these conditions are easily distinguished by clinical examination. Close inspection of the direction of the prolapsed tissue folds will reveal that in the case of full-thickness rectal prolapse, the folds are always concentric, whereas hemorrhoidal tissue or rectal mucosa develops radial invaginations.
Full inspection of the perineum and complete anorectal examination is equally important. A patulous anus with diminished sphincter tone is usually identified. Proctoscopy reveals a solitary rectal ulcer on the anterior surface of the rectum in 10% to 15% of cases. In the event that the prolapse is still elusive, patients can be asked to photo-graph the prolapse at home. Twenty to thirty-five percent of patients with rectal prolapse report urinary incontinence, and about 15% to 30% have significant vaginal vault prolapse.9,20 These symptoms require evaluation, and potentially, multidisciplinary surgical intervention.
2. Additional tests such as a defecography, colonoscopy, barium enema, and urodynamics can be used selectively to define the diagnosis and identify other important pathology. Grade of Recommendation: Strong recommendation based on moderate quality evidence 1B
If the prolapse cannot be produced during the physical examination, then a defecography may reveal the problem. Defecography may also reveal associated defects such as cystocele, vaginal vault prolapse, and enterocele that may, depending on symptoms, require treatment as well.21,22 Although uncommon, a neoplasm may form the lead point for a rectal intussusception.23 For this reason, and because this problem often occurs in the older population, colonoscopy should be performed based on existing guide-lines of appropriate screening for colorectal cancer. A significant finding on colonoscopic inspection may change the operative approach. For those patients who also have symptoms of vaginal prolapse or urinary incontinence, urodynamics and urogynecologic examination should be considered because surgical intervention may be needed for both the anterior and posterior compartments of the pelvis.24 –26
3. Physiologic testing may be useful to assess functional disorders associated with rectal prolapse, such as constipation or fecal incontinence. Grade of Recommendation: Weak recommendation based on low quality evidence 2C
Do Anorectal physiology studies rarely change the operative strategy for rectal prolapse, but they can often guide treatment for associated functional abnormalities, in particular, in the postoperative period. Patients will often present with rectal prolapse in the setting of lifelong severe constipation. These patients require special consideration in accordance with the ASCRS constipation practice pa-rameter.27 Anorectal physiology testing to assess for pelvic floor dyssynergia and a transit study to rule out colonic inertia should be considered in these situations. Patients with pelvic floor dyssynergia may benefit from postoperative biofeedback , and those who have evidence of surgically amenable slow-transit constipation, and are continent, may be candidates for subtotal colectomy in addition to a rectopexy.28
Chronic dilation of the anal sphincter with diminished internal anal sphincter pressures is a common finding and can lead to fecal incontinence. Again, the evaluation of these patients should be in accordance with the ASCRS practice parameter for fecal incontinence29 and may include endorectal ultrasound to evaluate sphincter defects, and anorectal manometry and pudendal nerve testing, as well. The finding of increased nerve conduction periods (nerve damage) may have postoperative prognostic significance for continence; patients with evidence of nerve damage may have a higher rate of incontinence following surgical correction of the prolapse, although more studies are required to confirm this.30 –32 In general, patients with fecal incontinence secondary to rectal prolapse will have improvement in their symptoms once the prolapse is treated. Unfortunately, in most studies, neither preoperative manometric findings nor nerve conduction velocities have served as reliable predictors of postoperative func-tion.33 Decreased anal squeeze or resting pressures may predate the actual development of the prolapse and con-tribute to the development of the condition.
Nonoperative Management
1. Although many patients who present with rectal pro-lapse are older and have multiple comorbidities, there is little nonoperative treatment available for symptomatic rectal prolapse. Grade: Weak recommendation based on low-quality evidence 2C
Addressing symptoms of constipation using fiber and stool softeners may be of use.34 Table sugar has been used to reduce incarcerated rectal prolapse by absorbing the edema of the rectal , thus making it easier to reduce.35 However, this does not definitively treat the condition. There are no studies that compare surgical and medical management of rectal prolapse.
Operations for Rectal Prolapse
Surgery is the mainstay for treatment of rectal prolapse. However, the number of procedures described in the literature both historically and in recent times continues to increase. Operative repairs include anal encirclement (historical interest only), mucosal resection, perineal proctosigmoidectomy, anterior resection with or without rectopexy, suture rectopexy alone, and a host of procedures involving the use of synthetic meshes affixed to the presacral fascia. Two predominant general approaches, abdominal and perineal, are considered in the operative re-pair of rectal prolapse. The surgical approach is dictated by the comorbidities of the patient, the surgeon’s preference and experience, and the patient’s age and bowel function.36 Although numerous operative approaches to rectal pro-lapse are described using both abdominal and perineal techniques, only a few are actually routinely advocated, and many are of historical interest only. Discussed here are procedures in common practice and routinely reported on in the literature.
Abdominal Procedures for Rectal Prolapse
1. In patients with acceptable risk, procedures incorporating trans-abdominal rectal fixation are typically the procedure of choice for the treatment of rectal prolapse. Grade of Recommendation: Strong recommendation based on moderate quality evidence 1B
In general, it is believed that the perineal approach results in less perioperative morbidity and pain, and a reduced length of hospital stay. However, recurrence rates that are 4 times higher than those for abdominal operations and worse functional outcome as a result of resection of the rectum have prevented this approach from becoming the procedure of choice.11,34,37 Abdominal operations generally have superior overall results and have become the preferred treatment for younger and healthier patients. However, morbidity and mortality is slightly higher with an abdominal approach, making the consideration of patient comorbidities essential in deciding the appropriate repair.33,34
Suture Rectopexy
1. Rectopexy is a key component in the abdominal approach to rectal prolapse. Grade of Recommendation: Strong recommendation based on low-quality evidence 1C
The fixation of the rectum in the pelvis with suture, first described by Cutait38 in 1959, aims to correct the telescoping of the redundant bowel and causes fixation of the rectum from the resultant scarring and fibrosis. The recurrence rates for suture rectopexy are generally re-ported to be from 3% to 9%.39–43 Rectopexy can also pro-duce new-onset or worsened constipation. Fifteen percent of patients experience constipation for the first time fol-lowing rectopexy, and at least 50% of those who are constipated preoperatively are made worse.44 The precise etiology of constipation is unclear. Mechanical as well as functional reasons for constipation should be considered.
2. A sigmoid resection may be added to rectopexy in patients with prolapse and preoperative constipation, but it is not necessary in those without constipation. Grade of Recommendation: Strong recommendation based on moderate-quality evidence 1B
Resection rectopexy is a technique first described by Frykman and Goldberg in 196945 and popularized in the United States in the past 30 years. The appeal of the procedure includes the lack of artificial mesh, ease of operation, and resection of “redundant” sigmoid colon. Recurrence rates are low, ranging from 2% to 5%, and major complication rates range from 0% to 20% and relate either to obstruction or anastomotic leak. The addition of sigmoid-ectomy to the operation was felt to be associated with a lower recurrence rate and improved functional outcome with a minimal increase in morbidity.46,47 It seems to re-duce constipation significantly in those who report this symptom preoperatively in some studies.34,46,48 Others have argued that sigmoidectomy is an inadequate operation for a chronic motility problem that affects the entire bowel, and those patients should be formally evaluated preoperatively and subtotal colectomy recommended if colonic inertia is detected. Although some patients who report incontinence before surgery will have an improvement in symptoms even after a sigmoid resection, resolution of fecal incontinence is less common if sigmoid resection is performed.34 There is increasing evidence that sigmoid resection may not be necessary in those who re-port no history of constipation and whose predominant complaint is fecal incontinence. This particular patient group does not seem to be predisposed to future constipation.49
3. Division of the lateral stalks during rectal dissection may worsen symptoms of constipation postoperatively, but it is associated with decreased recurrence rates. Grade of Recommendation: Weak recommendation based on moderate-quality evidence 2B
Independent of the technique used to perform the rectopexy, the division of the lateral stalks during the rectal dissection has been associated with worsening constipation.6,34,43,47,50 –53 It was theorized that the denervation of the rectum from the neural efferents thought to reside in the lateral ligaments was the cause of this complication. As a result, a revised version of the resection rectopexy advised preservation of the lateral stalks and unilateral fastening of the rectal mesentery to the sacrum at the level of the sacral promontory. However, multiple other studies examining the onset of constipation after preservation of the lateral stalks noted constipation in 18% to 89% of patients in comparison with 14% to 48% of those patients with lateral stalks divided. Furthermore, although there can be some improvement in constipation with preservation of the lateral ligaments, recurrence rates are found to be in-creased.34,51,53
Mesh Rectopexy
1. The Ripstein procedure with fixation of mesh from the anterior rectal wall to the sacral promontory after posterior mobilization may be used for treatment of rectal prolapse, but it is associated with higher morbidity. Grade of Recommendation: Strong recommendation based on low-quality evidence 1C
Prosthetic materials have long been used to affix the rectum to the sacrum to treat rectal prolapse. The Ripstein repair54 (and its many iterations) involves placement of a prosthetic mesh around the mobilized rectum with attach-ment of the mesh to the presacral fascia below the sacral promontory.13 Recurrence rates for this procedure range from 2.3% to 5%. After mobilization of the rectum, Rip-stein originally described using a band of rectangular mesh placed around the anterior aspect of the rectum at the level of the peritoneal reflection. Sutures were used to secure the mesh to the rectum anteriorly and the rectum was pulled upward and posterior. Then, both sides of the mesh were sutured to the presacral fascia. Recurrence rates ranged from 4% to 10%, but complication rates were excessive, up to 50%, primarily because of the placement of a foreign material on the anterior rectal wall.55–57
Because of, including large-bowel obstruction, erosion of the mesh through the bowel, ureteral injury or fibrosis, small-bowel obstruction, rectovaginal fistula, and fecal impaction, Ripstein modified the technique with posterior fixation of the mesh to the sacrum with attachment of the ends of the mesh to the rectum laterally.58 Recurrence rates are similar. Subsequent postoperative morbidity rates are 20%, but most of these complications are minor. Mesh rectopexy results in significant improvement in fecal incontinence in 20% to 60% of patients.9
2. A modified Wells procedure using a variety of foreign materials for posterior fixation of the rectum may be used for treatment of rectal prolapse. Grade of Evidence: Weak recommendation based on moderate-quality evidence 2B
Wells originally described fixation of the rectum using an Ivalon sponge and transection of the lateral ligaments. He reported excellent results with minimal complica-tions.59 A randomized trial of Ivalon (polyvinyl alcohol) sponge vs suture rectopexy found comparable recurrence rates but increased complication rates and postoperative constipation in the Ivalon group and recommended that this technique be abandoned.43 The Ivalon sponge is no longer commercially available. However, the modified Wells technique using other materials such as polyester or polypropylene mesh60,61 continues to be popular, especially for laparoscopic approaches.
3. The ventral mesh rectopexy reduces constipation by avoiding posterolateral mobilization of the rectum and produces results similar to other abdominal approaches. Grade of Recommendation: Weak recommendation based on moderate-quality evidence 2B
D’Hoore and colleagues62 first described the ventral rectopexy repair and its potential advantage in avoiding postoperative constipation. The technique involves mobilization of the anterior wall of the rectum with fixation of mesh to the anterior wall and then fixation of the mesh to the sacrum. This is in contrast to the Orr-Loygue procedure, 63 where the rectum was mobilized both anteriorly and posteriorly, before fixation to the sacrum. A systematic review of 12 non-randomized case series of 728 patients undergoing ventral rectopexy reported a recurrence rate of 3.4, and a weighted decrease in the postoperative constipation rate was estimated to be 23%. However, new onset of constipation was also noted to be 14.4%.64
Anterior Resection
1. The use of anterior resection alone to treat rectal pro-lapse is associated with higher recurrence rates and significant operative and postoperative morbidity; it should not be considered as a first-line treatment. Grade of recommendation: Strong recommendation based on moderate-quality evidence 1B
Anterior resection was described as an alternative strategy to repair prolapse in 1955, and there are some advocates of the technique. Unfortunately, in several retrospective reviews, several shortcomings are evident. In one review of 113 patients, the recurrence rate continued to climb after 2, 5, and 10 years to 3%, 6%, and 12%, with an operative morbidity of 29%, including 3 anastomotic leaks.65 Another review confirmed that, with an average follow-up of 6 years, recurrence occurred in 7% of cases.50 A low pelvic anastomosis in those with borderline continence may cause complete loss of control. Careful selection of patients is necessary for this procedure, and, in general, given the slightly higher recurrence rates and lack of functional advantages, it is not widely practiced.
Adjunctive Operative Techniques for Abdominal Procedures
1. A minimally invasive approach to rectal prolapse by experienced surgeons compares favorably with an open repair. Grade of Evidence: Strong recommendation based on moderate-quality evidence 1B
All abdominal approaches to rectal prolapse have been performed laparoscopically over the past decade with essentially similar results.34,66 – 69 The indications for per-forming a laparoscopic procedure are primarily related to the indications for an abdominal approach; patients with-out previous abdominal surgery are excellent candidates, but prior pelvic surgery is not necessarily an exclusion criterion. The laparoscopic treatment of rectal prolapse was first described in 1992 and involved a rectopexy without sigmoid resection.70 Since that time, numerous series have demonstrated equivalent recurrence rates (4%– 8%) and morbidity (10%–33%) of the laparoscopic repair in comparison with open approaches, but clear benefits in terms of pain control, length of stay, and return of bowel func-tion.15,34,68 In addition, the feasibility of a minimally invasive approach for colorectal resective procedures has been demonstrated in the high-risk patient.71 Wound complication rates have also been found to be decreased in laparoscopic surgery for prolapse. Certainly, those who undergo rectopexy without resection are at very low risk for infection because only trocar incisions are needed. The actual surgical technique to perform laparoscopic rectopexy or resection is the same as that used for open repairs. The goals of surgery remain the same, to eradicate the full-thickness rectal prolapse, improve bowel function and continence, and minimize recurrence rates.15 However, recurrence rates should be judged in light of the length of follow-up, because a significant percentage of recurrences may occur several years after treatment.72–74
Recent applications of robotic surgery for colorectal conditions have focused on pelvic operations because of the ease of maintaining one field for the procedure. Only a few series have been published with small numbers of patients that have demonstrated equivalent outcomes compared with laparoscopic approaches. Disadvantages include longer operating time and cost. However, visualization and ease of suturing and tying account for much of the interest in this technique.75–78
Perineal Operations for Rectal Prolapse
1. Patients with a short, full-thickness rectal prolapse can be treated with a mucosal sleeve resection; but, for a longer prolapse, it is associated with a higher recurrence rate compared with abdominal approaches. Grade of Recommendation: Strong recommendation based on low-quality evidence 1C
For patients with a short, full-thickness rectal prolapse or a mucosal prolapse, a Delorme procedure can be per-formed. It involves a circumferential mucosal sleeve resection and imbrication of the muscularis layer with serial vertical sutures. Recurrence rates are higher than the abdominal approaches in the range of 10% to 15%.79–82 This procedure is advocated for those who are considered “high risk” for an abdominal procedure because of comorbidities or to avoid risk of nerve damage. Complications such as infection, urinary retention, bleeding, and fecal impaction occur in 4% to 12% of cases.79,82 Constipation and fecal incontinence improve following surgery, but urgency and tenesmus do occur. Although restoration of function is not uniform in the series surveyed, in one of the few studies reporting postoperative manometric findings, both mean resting and squeeze pressures were significantly increased by the procedure.81
2. Patients with a full-thickness rectal prolapse who are not candidates for an abdominal operation may be treated with a perineal rectosigmoidectomy but are susceptible to higher recurrence rates in comparison with abdominal approaches. Grade of Recommendation: Strong recommendation based on low-quality evidence 1C
Perineal rectosigmoidectomy involves the full-thickness resection of the rectum and sigmoid colon via the anus with a coloanal anastomosis by the use of sutures or a stapling device. This operation can be performed without general anesthesia, involves a shorter hospital stay, and has lower complication rates (10%), which include bleeding from the staple or suture line, pelvic abscess, and, rarely, an anastomotic leak. As a result, patients undergoing perineal proctosigmoidectomy are generally older with significantly more comorbidities than those who are considered for abdominal repair.11,83 However, recurrence rates have been reported to be as high as 16% to 30%.11,83– 86 Other studies have shown that the use of levatoroplasty to treat levator diastasis can reduce recurrence rates from 21% to 7%.87,88 Only one small randomized controlled trial (n 20) has compared perineal rectosigmoidectomy with an abdominal approach. The recurrence rate was 10% for the perineal group vs 0% for the abdominal group.37
The practice parameters set forth in this document have been developed from sources believed to be reliable. The American Society of Colon and Rectal Surgeons makes no warranty, guarantee, or representation whatsoever as to the absolute validity or sufficiency of any parameter included in this document, and the Society assumes no responsibility for the use of the material contained.
APPENDIX A: CONTRIBUTING MEMBERS OF THE ASCRS STANDARDS COMMITTEE
Donald Buie, M.D., Chair; Janice Rafferty, M.D., Co-Chair; Farshid Araghizadeh, M.D.; Robin Boushey, M.D.; Srihdhar Chalasani, M.D.; George Chang, M.D.; Robert Cima, M.D.; Gary Dunn, M.D.; Daniel Feingold, M.D.; Phillip Fleshner, M.D.; Daniel Geisler, M.D.; Jill Jenua, M.D.; Sharon Gregorcyk, M.D.; Daniel Herzig, M.D.; An-dreas Kaiser, M.D.; Ravin Kumar, M.D.; David Larson, M.D.; Stephen Mills, M.D.; John Monson, M.D.; W. Brian Perry, M.D.; P. Terry Phang, M.D.; David Rivadeneira, M.D.; Howard Ross, M.D.; Peter Senatore, M.D.; Elin Sigurdson, M.D.; Thomas Stahl, M.D.; Scott Steele, M.D.; Scott Strong, M.D.; Charles Ternent, M.D.; Judith Trudel, M.D.; Madhulika Varma, M.D.; and Martin Weiser, M.D.
REFERENCES
1. Broden B, Snellman B. Procidentia of the rectum studied with cineradiography: a contribution to the discussion of causative mechanism. Dis Colon Rectum. 1968;11:330 –347.
2. Kuijpers HC. Treatment of complete rectal prolapse: to narrow, to wrap, to suspend, to fix, to encircle, to plicate or to resect?World J Surg. 1992;16:826 – 830.
3. Nicholls RJ. Rectal prolapse and the solitary ulcer syndrome. Ann Ital Chir. 1994;65:157–162.
4. Parks AG, Swash M, Urich H. Sphincter denervation in anorec-tal incontinence and rectal prolapse. Gut. 1977;18:656 – 665.
5. Roig JV, Buch E, Alos R, et al. Anorectal function in patients with complete rectal prolapse: differences between continent and incontinent individuals. Rev Esp Enferm Dig. 1998;90:794 –805.
6. Yakut M, Kaymakcioglu N, Simsek A, Tan A, Sen D. Surgical treatment of rectal prolapse: a retrospective analysis of 94 cases. Int Surg. 1998;83:53–55.
7. Gourgiotis S, Baratsis S. Rectal prolapse. Int J Colorectal Dis. 2007;22:231–243.
8. Kairaluoma MV, Kellokumpu IH. Epidemiologic aspects of complete rectal prolapse. Scand J Surg. 2005;94:207–210.
9. Madiba TE, Baig MK, Wexner SD. Surgical management of rec-tal prolapse. Arch Surg. 2005;140:63–73.
10. Marceau C, Parc Y, Debroux E, Tiret E, Parc R. Complete rectal prolapse in young patients: psychiatric disease a risk factor of poor outcome. Colorectal Dis. 2005;7:360 –365.
11. Kim DS, Tsang CB, Wong WD, Lowry AC, Goldberg SM, Mad-off RD. Complete rectal prolapse: evolution of management and results. Dis Colon Rectum. 1999;42:460 – 469.
12. Madoff RD, Mellgren A. One hundred years of rectal prolapse surgery. Dis Colon Rectum. 1999;42:441– 450.
13. Schultz I, Mellgren A, Dolk A, Johansson C, Holmstrom B. Long-term results and functional outcome after Ripstein rectopexy. Dis Colon Rectum. 2000;43:35– 43.
14. Schultz I, Mellgren A, Oberg M, Dolk A, Holmstrom B. Whole gut transit is prolonged after Ripstein rectopexy. Eur J Surg. 1999;165:242–247.
15. Senagore AJ. Management of rectal prolapse: the role of laparo-scopic approaches. Semin Laparosc Surg. 2003;10:197–202.
16. Glasgow SC, Birnbaum EH, Kodner IJ, Fleshman JW, Dietz DW. Preoperative anal manometry predicts continence after perineal proctectomy for rectal prolapse. Dis Colon Rectum. 2006;49: 1052–1058.
17. Snooks SJ, Henry MM, Swash M. Anorectal incontinence and rectal prolapse: differential assessment of the innervation to pu-borectalis and external anal sphincter muscles. Gut. 1985;26: 470 – 476.
18. Guyatt G, Gutermen D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest. 2006;129:174 –181.
19. Altman D, Zetterstrom J, Schultz I, et al. Pelvic organ prolapse and urinary incontinence in women with surgically managed rectal prolapse: a population-based case-control study. Dis Co-lon Rectum. 2006;49:28 –35.
20. Gonzalez-Argente FX, Jain A, Nogueras JJ, Davila GW, Weiss EG, Wexner SD. Prevalence and severity of urinary incontinence and pelvic genital prolapse in females with anal incontinence or rectal prolapse. Dis Colon Rectum. 2001;44:920 –926.
21. Pescatori M, Spyrou M, Pulvirenti d’Urso A. A prospective eval-uation of occult disorders in obstructed defecation using the ‘iceberg diagram.’ Colorectal Dis. 2006;8:785–789.
22. Renzi A, Izzo D, Di Sarno G, et al. Cinedefecographic findings in patients with obstructed defecation syndrome: a study in 420 cases. Minerva Chir. 2006;61:493– 499.
23. Bounovas A, Polychronidis A, Laftsidis P, Simopoulos C. Sig-moid colon cancer presenting as complete rectal prolapse. Colo-rectal Dis. 2007;9:665– 666.
24. Lim M, Sagar PM, Gonsalves S, Thekkinkattil D, Landon C. Surgical management of pelvic organ prolapse in females: functional outcome of mesh sacrocolpopexy and rectopexy as a com-bined procedure. Dis Colon Rectum. 2007;50:1412–1421.
25. Mellgren A, Johansson C, Dolk A, et al. Enterocele demon-strated by defaecography is associated with other pelvic floor disorders. Int J Colorectal Dis. 1994;9:121–124.
26. Sagar PM, Thekkinkattil DK, Heath RM, Woodfield J, Gon-salves S, Landon CR. Feasibility and functional outcome of laparoscopic sacrocolporectopexy for combined vaginal and rectal prolapse. Dis Colon Rectum. 2008;51:1414 –1420.
27. Ternent CA, Bastawrous AL, Morin NA, Ellis CN, Hyman NH, Buie WD. Practice parameters for the evaluation and manage-ment of constipation. Dis Colon Rectum. 2007;50:2013–2022.
28. Watts JD, Rothenberger DA, Buls JG, Goldberg SM, Nivatvongs
S. The management of procidentia: 30 years’ experience. Dis Colon Rectum. 1985;28:96 –102.
29. Tjandra JJ, Dykes SL, Kumar RR, et al. Practice parameters for the treatment of fecal incontinence. Dis Colon Rectum. 2007;50: 1497–1507.
30. Birnbaum EH, Stamm L, Rafferty JF, Fry RD, Kodner IJ, Flesh-man JW. Pudendal nerve terminal motor latency influences sur-gical outcome in treatment of rectal prolapse. Dis Colon Rectum. 1996;39:1215–1221.
31. Johansen OB, Wexner SD, Daniel N, Nogueras JJ, Jagelman DG. Perineal rectosigmoidectomy in the elderly. Dis Colon Rectum. 1993;36:767–772.
32. Schultz I, Mellgren A, Nilsson BY, Dolk A, Holmstrom B. Pre-operative electrophysiologic assessment cannot predict continence after rectopexy. Dis Colon Rectum. 1998;41:1392–1398.
33. Felt-Bersma RJ, Tiersma ES, Cuesta MA. Rectal prolapse, rectal intussusception, rectocele, solitary rectal ulcer syndrome, and enterocele. Gastroenterol Clin North Am. 2008;37:645– 668.
34. Tou S, Brown SR, Malik AI, Nelson RL. Surgery for complete rectal prolapse in adults. Cochrane Database Syst Rev. 2008: CD001758.
35. Myers JO, Rothenberger DA. Sugar in the reduction of incarcer-ated prolapsed bowel: report of two cases. Dis Colon Rectum. 1991;34:416 – 418.
36. Brown AJ, Anderson JH, McKee RF, Finlay IG. Strategy for se-lection of type of operation for rectal prolapse based on clinical criteria. Dis Colon Rectum. 2004;47:103–107.
37. Deen KI, Grant E, Billingham C, Keighley MR. Abdominal re-section rectopexy with pelvic floor repair versus perineal rec-tosigmoidectomy and pelvic floor repair for full-thickness rectal prolapse. Br J Surg. 1994;81:302–304.
38. Cutait D. Sacro-promontory fixation of the rectum for complete rectal prolapse. Proc R Soc Med. 1959;52:105.
39. Briel JW, Schouten WR, Boerma MO. Long-term results of su-ture rectopexy in patients with fecal incontinence associated with incomplete rectal prolapse. Dis Colon Rectum. 1997;40: 1228 –1232.
40. Carter AE. Rectosacral suture fixation for complete rectal pro-lapse in the elderly, the frail and the demented. Br J Surg. 1983; 70:522–523.
41. Graf W, Karlbom U, Pahlman L, Nilsson S, Ejerblad S. Functional results after abdominal suture rectopexy for rectal pro-lapse or intussusception. Eur J Surg. 1996;162:905–911.
42. Khanna AK, Misra MK, Kumar K. Simplified sutured sacral rectopexy for complete rectal prolapse in adults. Eur J Surg. 1996; 162:143–146.
43. Novell JR, Osborne MJ, Winslet MC, Lewis AA. Prospective ran-domized trial of Ivalon sponge versus sutured rectopexy for full-thickness rectal prolapse. Br J Surg. 1994;81:904 –906.
44. Aitola PT, Hiltunen KM, Matikainen MJ. Functional results of operative treatment of rectal prolapse over an 11-year period: emphasis on transabdominal approach. Dis Colon Rectum. 1999; 42:655– 660.
45. Frykman HM, Goldberg SM. The surgical treatment of rectal procidentia. Surg Gynecol Obstet. 1969;129:1225–1230.
46. Luukkonen P, Mikkonen U, Jarvinen H. Abdominal rectopexy with sigmoidectomy vs. rectopexy alone for rectal prolapse: a prospective, randomized study. Int J Colorectal Dis. 1992;7:219 –222.
47. Sayfan J, Pinho M, Alexander-Williams J, Keighley MR. Sutured posterior abdominal rectopexy with sigmoidectomy compared with Marlex rectopexy for rectal prolapse. Br J Surg. 1990;77: 143–145.
48. McKee RF, Lauder JC, Poon FW, Aitchison MA, Finlay IG. A prospective randomized study of abdominal rectopexy with and without sigmoidectomy in rectal prolapse. Surg Gynecol Obstet. 1992;174:145–148.
49. Hsu A, Brand MI, Saclarides TJ. Laparoscopic rectopexy without resection: a worthwhile treatment for rectal prolapse in patients without prior constipation. Am Surg. 2007;73:858 – 861.
50. Cirocco WC, Brown AC. Anterior resection for the treatment of rectal prolapse: a 20-year experience. Am Surg. 1993;59:265–269.
51. Mollen RM, Kuijpers JH, van Hoek F. Effects of rectal mobiliza-tion and lateral ligaments division on colonic and anorectal function. Dis Colon Rectum. 2000;43:1283–1287.
52. Scaglia M, Fasth S, Hallgren T, Nordgren S, Oresland T, Hulten
L. Abdominal rectopexy for rectal prolapse: influence of surgical technique on functional outcome. Dis Colon Rectum. 1994;37: 805– 813.
53. Speakman CT, Madden MV, Nicholls RJ, Kamm MA. Lateral ligament division during rectopexy causes constipation but pre-vents recurrence: results of a prospective randomized study. Br J Surg. 1991;78:1431–1433.
54. Ripstein CB, Lanter B. Etiology and surgical therapy of massive prolapse of the rectum. Ann Surg. 1963;157:259 –264.
55. Gordon PH, Hoexter B. Complications of the Ripstein procedure. Dis Colon Rectum. 1978;21:277–280.
56. Kupfer CA, Goligher JC. One hundred consecutive cases of complete prolapse of the rectum treated by operation. Br J Surg. 1970;57:482– 487.
57. Roberts PL, Schoetz DJ Jr, Coller JA, Veidenheimer MC. Rip-stein procedure. Lahey Clinic experience: 1963–1985. Arch Surg. 1988;123:554 –557.
58. McMahan JD, Ripstein CB. Rectal prolapse: an update on the rectal sling procedure. Am Surg. 1987;53:37– 40.
59. Wells C. New operation for rectal prolapse. Proc R Soc Med. 1959;52:602– 603.
60. Dulucq JL, Wintringer P, Mahajna A. Clinical and functional outcome of laparoscopic posterior rectopexy (Wells) for full-thickness rectal prolapse: a prospective study. Surg Endosc. 2007; 21:2226 –2230.
61. Madbouly KM, Senagore AJ, Delaney CP, Duepree HJ, Brady KM, Fazio VW. Clinically based management of rectal prolapse. Surg Endosc. 2003;17:99 –103.
62. D’Hoore A, Penninckx F. Laparoscopic ventral recto(col-po)pexy for rectal prolapse: surgical technique and outcome for 109 patients. Surg Endosc. 2006;20:1919 –1923.
63. Loygue J, Nordlinger B, Cunci O, Malafosse M, Huguet C, Parc
R. Rectopexy to the promontory for the treatment of rectal pro-lapse: report of 257 cases. Dis Colon Rectum. 1984;27:356 –359.
64. Samaranayake CB, Luo C, Plank AW, Merrie AE, Plank LD, Bissett IP. Systematic review on ventral rectopexy for rectal pro-lapse and intussusception. Colorectal Dis. 2010;12:504 –512.
65. Schlinkert RT, Beart RW Jr, Wolff BG, Pemberton JH. Anterior resection for complete rectal prolapse. Dis Colon Rectum. 1985; 28:409 – 412.
66. Boccasanta P, Venturi M, Reitano MC, et al. Laparotomic vs. laparoscopic rectopexy in complete rectal prolapse. Dig Surg. 1999;16:415– 419.
67. Kariv Y, Delaney CP, Casillas S, et al. Long-term outcome after laparoscopic and open surgery for rectal prolapse: a case-control study. Surg Endosc. 2006;20:35– 42.
68. Purkayastha S, Tekkis P, Athanasiou T, et al. A comparison of open vs. laparoscopic abdominal rectopexy for full-thickness rectal prolapse: a meta-analysis. Dis Colon Rectum. 2005;48: 1930 –1940.
69. Solomon MJ, Young CJ, Eyers AA, Roberts RA. Randomized clinical trial of laparoscopic versus open abdominal rectopexy for rectal prolapse. Br J Surg. 2002;89:35–39.
70. Berman IR. Sutureless laparoscopic rectopexy for procidentia: technique and implications. Dis Colon Rectum. 1992;35:689 –693.
71. Plocek MD, Geisler DP, Glennon EJ, Kondylis P, Reilly JC. Laparoscopic colorectal surgery in the complicated patient. Am J Surg. 2005;190:882– 885.
72. DiGiuro G, Ignjatovic D, Brogger J, Bergamaschi R. How accu-rate are published recurrence rates after rectal prolapse surgery?A meta-analysis of individual patient data. Am J Surg. 2006;191: 773–778.
73. Raftopoulos Y, Senagore AJ, Di Giuro G, Bergamaschi R. Recurrence rates after abdominal surgery for complete rectal prolapse: a multicenter pooled analysis of 643 individual patient data. Dis Colon Rectum. 2005;48:1200 –1206.
74. Byrne CM, Smith SR, Solomon MJ, Young JM, Eyers AA, Young CJ. Long-term functional outcomes after laparoscopic and open rectopexy for the treatment of rectal prolapse. Dis Colon Rectum. 2008;51:1597–1604.
75. Ayav A, Bresler L, Hubert J, Brunaud L, Boissel P. Robotic-as-sisted pelvic organ prolapse surgery. Surg Endosc. 2005;19:1200 –1203.
76. Delaney CP, Lynch AC, Senagore AJ, Fazio VW. Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum. 2003;46:1633–1639.
77. Heemskerk J, de Hoog DE, van Gemert WG, Baeten CG, Greve JW, Bouvy ND. Robot-assisted vs conventional laparoscopic rectopexy for rectal prolapse: a comparative study on costs and time. Dis Colon Rectum. 2007;50:1825–1830.
78. Munz Y, Moorthy K, Kudchadkar R, et al. Robotic assisted rectopexy. Am J Surg. 2004;187:88 –92.
79. Lieberth M, Kondylis LA, Reilly JC, Kondylis PD. The Delorme repair for full-thickness rectal prolapse: a retrospective review. Am J Surg. 2009;197:418 – 423.
80. Senapati A, Nicholls RJ, Thomson JP, Phillips RK. Results of Delorme’s procedure for rectal prolapse. Dis Colon Rectum. 1994;37:456 – 460.
81. Tsunoda A, Yasuda N, Yokoyama N, Kamiyama G, Kusano M. Delorme’s procedure for rectal prolapse: clinical and physiolog-ical analysis. Dis Colon Rectum. 2003;46:1260 –1265.
82. Watkins BP, Landercasper J, Belzer GE, et al. Long-term fol-low-up of the modified Delorme procedure for rectal prolapse. Arch Surg. 2003;138:498 –502.
83. Riansuwan W, Hull TL, Bast J, Hammel JP, Church JM. Comparison of perineal operations with abdominal operations for full-thickness rectal prolapse. World J Surg. 2010;34:1116 –1122.
84. Azimuddin K, Khubchandani IT, Rosen L, Stasik JJ, Riether RD, Reed JF III. Rectal prolapse: a search for the “best” operation. Am Surg. 2001;67:622– 627.
85. Pescatori M, Zbar AP. Tailored surgery for internal and external rectal prolapse: functional results of 268 patients operated upon by a single surgeon over a 21-year period*. Colorectal Dis. 2009; 11:410 – 419.
86. Altomare DF, Binda G, Ganio E, De Nardi P, Giamundo P, Pes-catori M. Long-term outcome of Altemeier’s procedure for rec-tal prolapse. Dis Colon Rectum. 2009;52:698 –703.
87. Chun SW, Pikarsky AJ, You SY, et al. Perineal rectosigmoidec-tomy for rectal prolapse: role of levatorplasty. Tech Coloproctol. 2004;8:3–9.
88. Habr-Gama A, Jacob CE, Jorge JM, et al. Rectal procidentia treatment by perineal rectosigmoidectomy combined with leva-tor ani repair. Hepatogastroenterology. 2006;53:213–217.