Practice Parameters for the Management of Perianal Abscess and Fistula-in-Ano
Scott R. Steele, M.D. • Ravin Kumar, M.D. • Daniel L. Feingold, M.D. • Janice L. Rafferty, M.D. • W. Donald Buie, M.D.
Prepared by the Standards Practice Task Force of the American Society of Colon and Rectal Surgeons
The American Society of Colon and Rectal Surgeons is dedicated to ensuring high quality patient care by advancing the science, prevention, and management of disorders and diseases of the colon, rectum, and anus. The Standards Committee is composed of Society members who are chosen because they have demonstrated expertise in the specialty of colon and rectal surgery. This Committee was created to lead international efforts in defining quality care for conditions related to the colon, rectum, and anus. This is accompanied by developing Clinical Practice Guidelines based on the best available evidence. These guidelines are inclusive, and not prescriptive. Their purpose is to provide information on which decisions can be made, rather than dictate a specific form of treatment. These guidelines are intended for the use of all practitioners, health care workers, and patients who desire information about the management of the conditions addressed by the topics covered in these guidelines.
It should be recognized that these guidelines should not be deemed inclusive of all proper methods of care or exclusive of methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding the propriety of any specific procedure must be made by the physician in light of all the circumstances presented by the individual patient.
STATEMENT OF THE PROBLEM
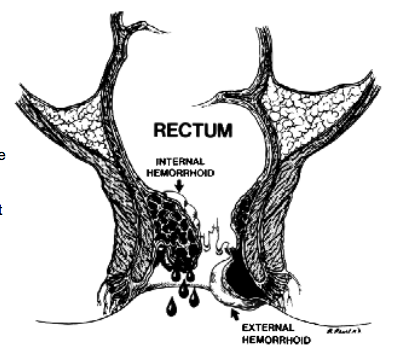
Anorectal abscess is a potentially debilitating condition most often originating from a cryptoglandular infection in the anal canal,1 and remains one of the more common anorectal conditions encountered in practice. Although the underlying pathogenesis is the same in the majority of patients, these abscesses are classified into perianal, ischiorectal, intersphincteric, and supralevator based on location.2 As such, patients may present with a variety of signs and symptoms ranging from fever, pain, tenderness, erythema, and a fluctuant mass to relatively normal external findings and deep-seated rectal pain.2
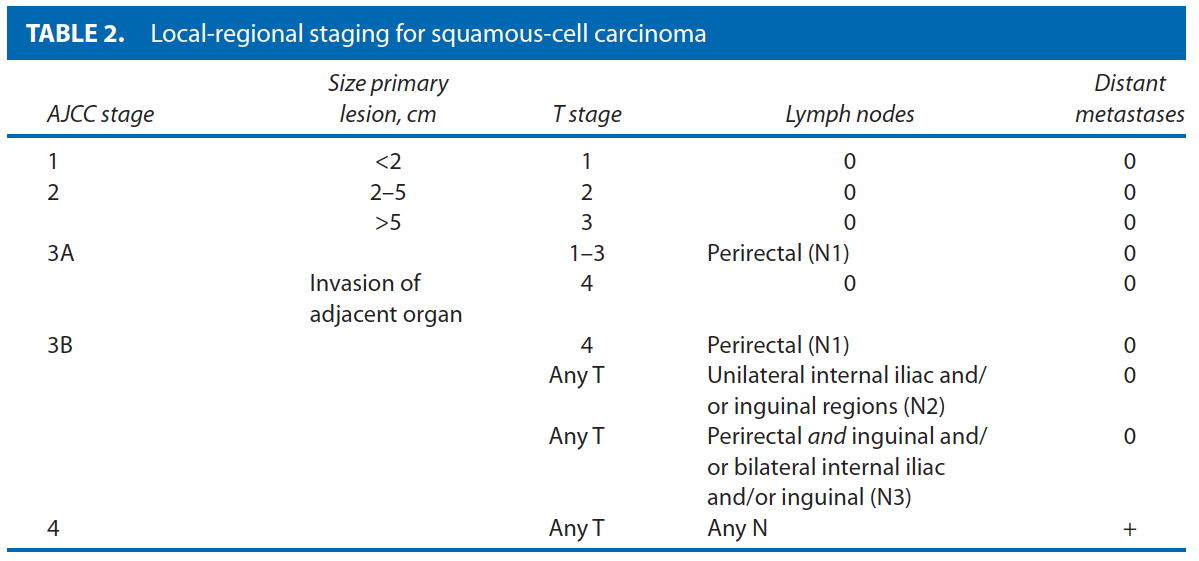
In approximately 30% to 50% of patients with an anorectal abscess, a persistent tract, or fistula-in-ano, develops, extending from the anal canal to the perineal skin.3,4 Unfortunately, there is no definitive way to predict who will develop one, or how to prevent one. Patients often report persistent purulent drainage or intermittent perianal swelling and tenderness followed by spontaneous discharge. Fistulas are categorized based on their anatomical course relative to the sphincter complex: intersphincteric, transsphincteric, suprasphincteric, and extrasphincteric.4 Fistulas can also be classified as “simple” or “complex,” with simple fistulas including low transsphincteric and intersphincteric fistulas that cross �30% of the external sphincter.5 Complex fistulas include high transsphincteric fistulas with or without a high blind tract, suprasphincteric and extrasphincteric fistulas, horseshoe fistulas, and those associated with inflammatory bowel disease, radiation, malignancy, preexisting incontinence, or chronic diarrhea, as well.6–8 Given the attenuated nature of the anterior sphincter complex in women, fistulas in this location deserve special consideration and may be considered complex as well. This practice parameter will focus on the evaluation and management of both perianal abscess and fis-tula-in-ano.
METHODOLOGY
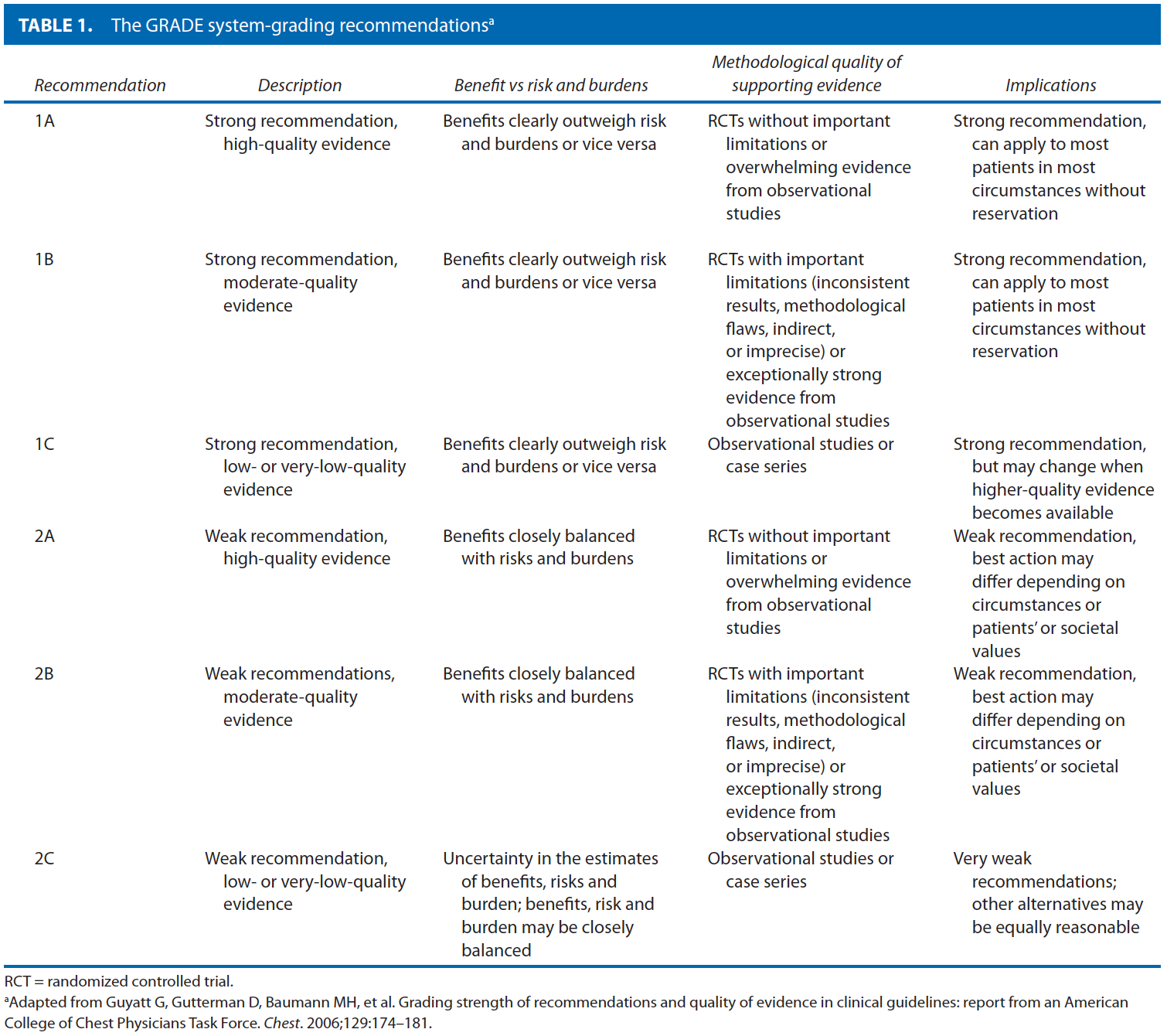
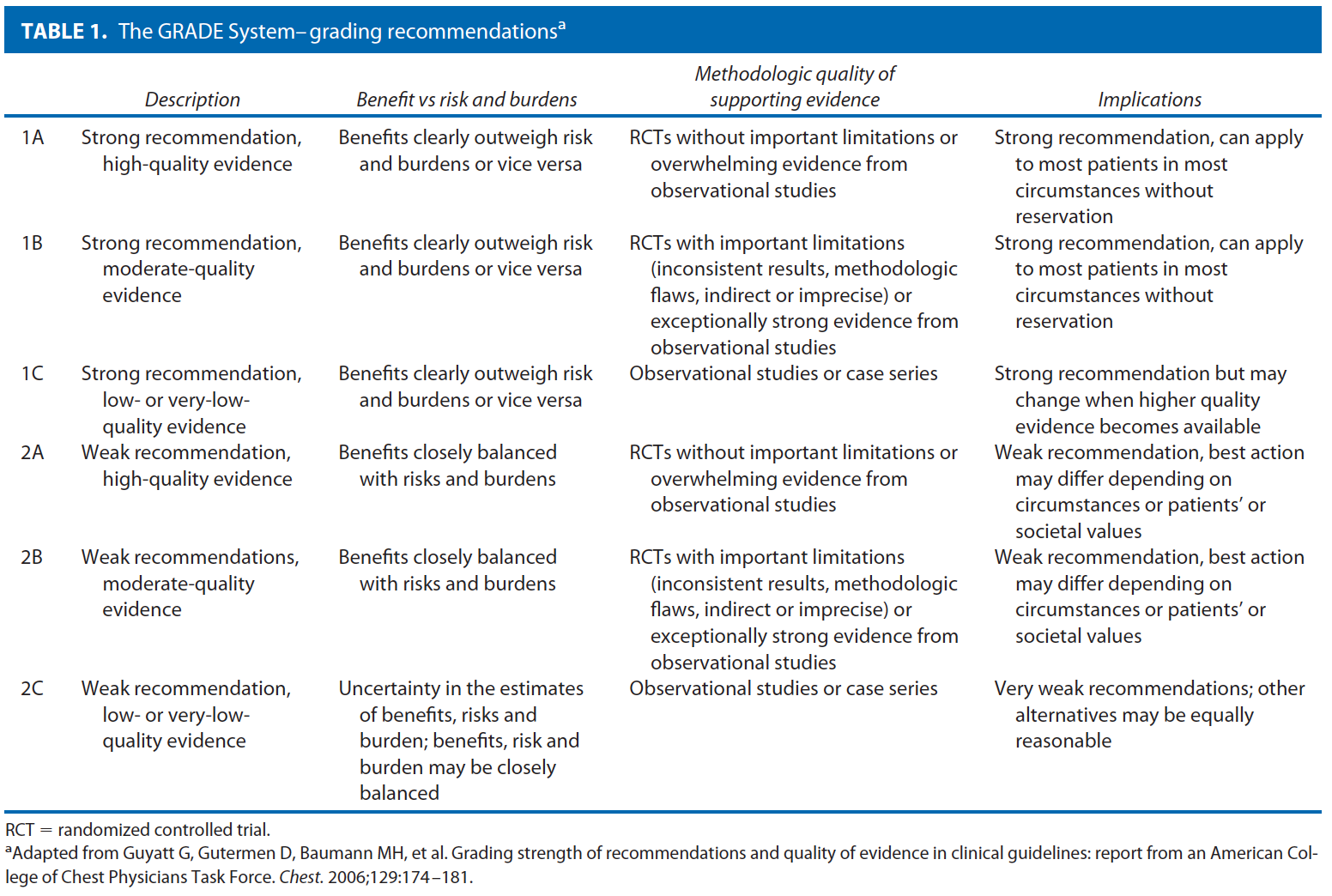
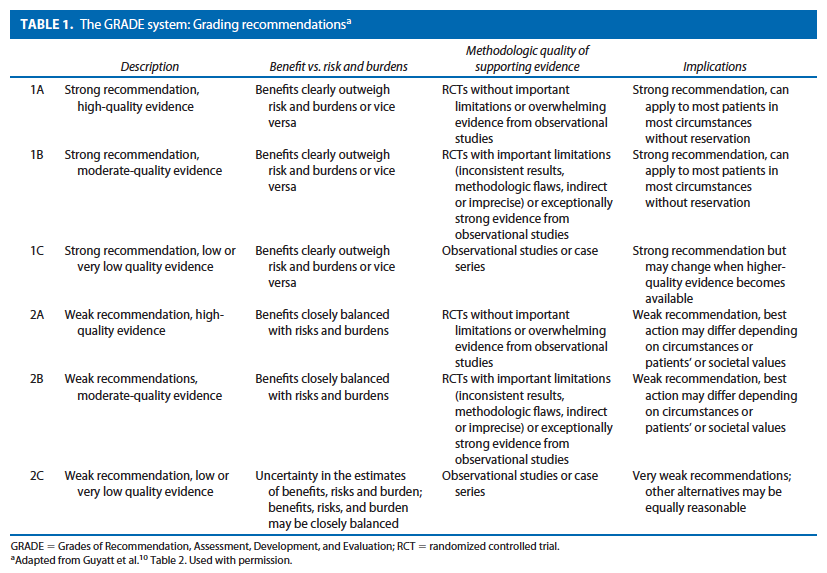
These guidelines are built on the last set of the American Society of Colon and Rectal Surgeons Practice Parameters for treatment of perianal abscess and fistula-in-ano published in 2005.9 An organized search of MEDLINE, PubMed, EMBASE, and the Cochrane Database of Collected Reviews was performed through February 2010. Key word combinations included abscess, fistula, fistula-in-ano, anal, rectal, perianal, perineal, rectovaginal, anovaginal, seton, fistula plug, fibrin glue, advancement flap, and Crohn’s disease. Directed searches of the embedded references from the primary articles were also performed in selected circumstances. Primary authors reviewed all English language manuscripts and studies of adults. Recommendations were formulated by the primary authors and reviewed by the entire Committee. The final grade of recommendation was performed using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) system10 and reviewed by the entire Standards Committee (Table 1).11
RECOMMENDATIONS
Initial Evaluation of Perianal Abscess and Fistula-in-Ano
- 1. A disease-specific history and physical examination should be performed, emphasizing symptoms, risk factors, location, and presence of secondary cellulitis or fistula-in-ano. Grade of Recommendation: Strong recommendation based on low-quality evidence 1C
The diagnosis of anorectal abscess is usually made based on the patient’s history and physical examination. It is important to distinguish anorectal abscess from other perianal suppurative processes such as hidradenitis suppurativa, infected skin furuncles, and infectious processes including herpes simplex virus, HIV, tuberculosis, syphilis, and actinomycosis.12 In addition, features suggestive of Crohn’s disease, including large skin tags or multiple fistu-las, require a more detailed workup and potentially additional medical therapy.13
On examination, a tender, fluctuant mass is almost always present with perianal and ischiorectal abscesses. Patients with intersphincteric or supralevator abscesses may have a paucity of external findings, with only pelvic or rectal tenderness or fluctuance on digital rectal examination. Careful inspection may detect the presence of other anorectal pathology or an external opening suggestive of a fis-tula-in-ano.14,15 Palpation of the perianal area, digital rectal examination, and careful probing of the tract(s) often aids in defining the presence and anatomy of the fistula. Anoscopy and sigmoidoscopy may be performed to try to visualize the internal opening of a fistula and other mucosal abnormalities such as proctitis secondary to Crohn’s disease. In general, laboratory evaluation is not necessary, with the exception of patients with systemic symptoms such as fever, serious underlying medical problems, or an unclear diagnosis.
- 2. Studies such as fistulography, endoanal ultrasound, CT scan, and MRI may be considered in selected patients to help define the anatomy of an anorectal abscess or fistula-in-ano and to guide management. Grade of Recommendation: Strong recommendation based on low-quality evidence 1C
Although anorectal abscess and fistula-in-ano are most commonly diagnosed and managed on the basis of clinical findings alone, adjunctive radiological studies can occasionally provide valuable information in complex tracts or recurrent disease. The vast majority of fistulas, however, do not require any imaging. Traditionally, fistulography was the method of choice.16 Reported accuracy rates as low as 16% have largely led to this test falling out of favor.17 Endoanal ultrasound is very effective for characterizing anorectal abscess and fistulas with accuracy rates as high as 80% to 89% for delineating fistula tracts, and is especially effective in identifying horseshoe abscess extensions.18 –20 Three-dimensional ultrasound techniques provide even better imaging, especially in patients with complex perianal sepsis or high-riding tracts.21 Combining 3-dimensional ultrasound with hydrogen peroxide injection through the external opening has demonstrated accuracy rates comparable to MRI, with close to 90% concordance.22–24 CT scan can be useful for patients with complex suppurative anorectal conditions, and is especially helpful in identifying supralevator abscesses, or for those patients who would otherwise be difficult to examine without anesthesia.25 In patients with Crohn’s disease who have perianal pathology, CT has proven reliable in helping to delineate fistulas and abscesses from isolated rectal inflammation.26
MRI with or without endoanal coils has reported accuracy rates of more than 90% for mapping fistula tracts and identifying the internal opening.27,28 The majority of studies comparing pelvic MRI with endoanal ultrasound have shown slightly higher20,22,29,30 rather than lower31,32 rates of sensitivity and accuracy— depending, in part, on operator experience (ultrasound) and patient population (ie, recurrent disease, abscess/fistula location, Crohn’s disease).
Perianal Abscess
- 1. Patients with acute anorectal abscess should be treated in a timely fashion with incision and drainage. Grade of Recommendation: Strong recommendation based on low-quality evidence 1C
The primary treatment of anorectal abscesses remains surgical drainage. In general, the incision should be kept as close as possible to the anal verge to minimize the length of a potential fistula, while still providing adequate drainage. With an adequately sized elliptical incision, postoperative wound packing is usually not necessary. A variation of incision and drainage uses a small latex catheter (eg, 10 –14F Pezzer catheter) placed into the abscess cavity with the use of local anesthesia and a small stab incision. The catheter is removed when the abscess drainage stops and the cavity has closed down around the catheter (usually 3–10 days).33,34
After simple incision and drainage, the overall recurrence rate ranges from 3% to 44%, depending on the abscess location and the length of follow up.35,36 Additional factors associated with recurrence and the need for early repeat drainage include incomplete initial drainage, failure to break up loculations within the abscess, missed abscess, and undiagnosed fistula.37 Horseshoe abscesses have been associated with especially high rates of persistence and recurrence ranging between 18% and 50%,37,38 and often require multiple operations before definitive healing.39
- 2. Antibiotics have a limited role in the treatment of uncomplicated anorectal abscess. Grade of Recommendation: Strong recommendation based on moderatequality evidence 1B
- 3. Antibiotics may be considered in patients with significant cellulitis, underlying immunosuppression, or concomitant systemic illness. Grade of Recommendation: Weak recommendation based on low-quality evidence 2C
In general, the addition of antibiotics to routine incision and drainage of uncomplicated anorectal abscess does not improve healing time or reduce recurrences, and it is therefore not indicated.40–42 However, limited data suggest that antibiotics be considered for use in patients with extensive cellulitis, systemic symptoms, or failure to improve with drainage alone.43 In patients with underlying immunosuppression, the data also suggest that antibiotics may play a role. Although patients with a higher absolute neutrophil count (�1000/mm3) and fluctuance on examination demonstrate higher resolution rates with incision and drainage, patients with lower neutrophil counts (ANC �500 –1000/mm3) and/or lack of fluctuance on examination have been successfully treated with antibiotics alone in 30% to 88%.44–46
The emergence of community-acquired methicillin-resistant Staphylococcus aureus in otherwise routine anorectal abscesses47 raises the question whether wound culture is indicated after incision and drainage. Although wound culture is rarely helpful, it may be considered in cases of recurrent infection or nonhealing wounds. Patients with underlying HIV infection with either concomitant infections or atypical microbes, including tuberculosis48 may benefit from wound culture and targeted antibiotic treatment.
Finally, recent guidelines from the American Heart Association recommend preoperative antibiotics before incision and drainage of infected tissue in patients with prosthetic valves, previous bacterial endocarditis, congenital heart disease, and heart transplant recipients with valve pathology. Unlike prior guidelines, antibiotic prophylaxis is no longer recommended in patients with routine mitral valve prolapse.49
Fistula-in-Ano
The goal in the treatment of fistula-in-ano is to obliterate the internal fistulous opening and any associated epitheli-alized tracks with minimal sphincter division. Thus, it is imperative to identify the internal opening and the course of all tracts relative to the sphincter muscles. Goodsall’s rule attempts to predict the location of the internal open-ing in relation to its external (secondary) opening. External openings posterior to a transverse line through the anal verge will open into the anal canal in the midline. Con-versely, anterior placed openings will run in a radial direc-tion, analogous to “spokes on a wheel.” Although Good-sall’s rule accurately predicts the location of the internal opening in 49% to 81% of patients, the location of the external opening can be a poor predictor of the location of a fistula, in particular, in patients with long fistula tracts, recurrent fistulas, or Crohn’s disease.14,50 –52 In addition to direct visualization and palpation, the surgeon must be familiar with adjunctive intraoperative measures, includ-ing hydrogen peroxide/methylene blue injection of the ex-ternal opening, to assist in the identification of tract origin, with reported success rates greater than 90% and 80%.50,51 In addition, the etiology should be determined. Approxi-mately 80% of fistulas are secondary to cryptoglandular infection, but other diagnoses such as Crohn’s, trauma, radiation, malignancy, or infection must be considered in fistulas with an unusual appearance or location.
Because no single technique is appropriate for the treatment of all fistulas-in-ano, treatment must be directed by the etiology and anatomy of the fistula, degree of symp-toms, patient comorbidities, and the surgeon’s experience. One should keep in mind the progressive tradeoff between the extent of operative sphincter division, postoperative healing rates, and functional compromise.
Treatment of a Simple Fistula-in-Ano
- 1. Simple anal fistulas may be treated by fistulotomy. The addition of marsupialization may improve the rate of wound healing. Grade of Recommendation: Strong recom-mendation based on moderate-quality evidence 1B
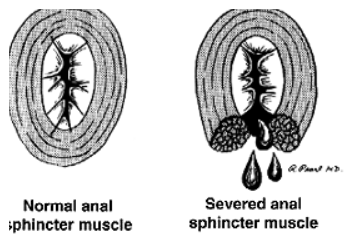
There is no universal answer to the question of how much muscle can be safely divided. Nevertheless, with proper patient selection, fistulotomy has been associated with success rates of 92% to 97%.53,54 Higher recurrence rates have been associated with complex fistulas, failure to identify the internal opening, and Crohn’s disease.53,55,56
Postoperative alterations in continence are reported in 0% to 73% of patients. This wide range is due to differences in the definition of incontinence, variable follow-up, and the degree of disturbance. Risk factors include preopera-tive incontinence, recurrent disease, female sex, complex fistulas, and prior fistula surgery.53,56 –58 The addition of marsupialization has also been associated with less postop-erative bleeding and accelerated wound healing by approx-imately 4 weeks.59,60 Limited data have shown that fistulectomy, in which the tract is resected, is associated with longer healing times, larger defects, and a higher risk of incontinence, although recurrence rates are similar when compared with fistulotomy.61,62
- 2. Concomitant fistulotomy with incision and drain-age may be considered in select patients with anorectal ab-scess and fistula. Grade of Recommendation: Weak recom-mendation based on moderate-quality evidence 2B
One of the more controversial topics in dealing with anorectal abscess is the role of primary fistulotomy at the time of initial incision and drainage. Proponents of this practice cite the infected crypt as the origin of the problem and believe that failure to address this leads to higher re-currence rates.35 Opponents counter with the higher rate of continence disturbances in patients undergoing con-comitant fistulotomy and the potential for unnecessary fistulotomy.3,63 Quah and colleagues performed a meta-analysis including 5 trials of 405 patients and demon-strated that sphincter division (via fistulotomy or fistulec-tomy) at the time of incision and drainage was associated with a significant decrease in recurrence (relative risk (RR) � 0.17, 95% CI 0.09 – 0.32, P � .001), but higher levels of continence disorders (RR � 2.46, 95% CI 0.75–8.06, P � .140).64
Thus, the utility of fistulotomy in conjunction with incision and drainage of an anorectal abscess remains con-troversial. The surgeon should weigh the possible de-creased recurrence rate in light of the potential increased risk of continence disturbances.
- 3. Simple anal fistulas may be treated with debride-ment and fibrin glue injection. Grade of Recommenda-tion: Weak recommendation based on low-quality evi-dence 2C
Fibrin glue has a number of advantages, including its ease of use, repeatability, and avoidance of sphincter divi-sion, especially in patients with a high risk of incontinence following fistulotomy. However, this must be weighed against the high failure rate. Retrospective and prospective cohort data for fibrin glue use in simple fistulas has dem-onstrated healing rates of 40% to 78%.65– 68 Simple low fistulas have a decreased rate of healing with fibrin glue compared with fistulotomy (50% (3/6) vs 100% (7/7), P � .06) with low rates of incontinence in both groups.
Treatment of Complex Fistula-in-Ano
In select patients, radiographic evaluation may be benefi-cial to identify an occult internal opening and secondary tracts or abscesses, or to help delineate the fistula’s rela-tionship to the sphincter complex.
- 1. Complex anal fistulas may be treated with de-bridement and fibrin glue injection. Grade of Recom-mendation: Weak recommendation based on low-qual-ity evidence 2C
In the randomized controlled trial by Lindsey et al,69 the authors compared fibrin glue with loose setons followed by flap repair for complex fistulas (n � 29). Fibrin glue was associated with higher healing rates (69% (9/13) vs 13% (2/16), P � .003), whereas incontinence rates were similar between the 2 groups (0/13 vs 2/16). Overall heal-ing rates in nonrandomized series using fibrin glue for complex disease has ranged from 10% to 67%.66 –71
Although fibrin glue therapy has a relatively low suc-cess rate in complex disease, for those that eventually heal, it does appear to be a durable repair. Furthermore, given the low morbidity associated with the procedure, it may be considered for initial therapy.
- 2. Anal fistula plug may be used for treatment of complex anal fistula disease. Grade of Recommendation: Weak recommendation based on moderate-quality evi-dence 2C
The bioprosthetic anal fistula plug is used to close the primary internal anal opening and serves as a matrix for the obliteration of the fistula tract. Although limited data have demonstrated success with the plug in up to 70% to 100% of low-lying fistulas, outcomes in complex disease have been less promising.72–75 In patients with Crohn’s disease, initial reports demonstrated closure rates of 80%,76 with the same group demonstrating persistent clo-sure in 83% of all types of complex fistulas at a median of 12 months.77
Unfortunately, most other studies have not been able to replicate those results, with the majority of studies re-porting �50%.72,73,78 – 80 Lower success rates are associ-ated with longer follow-up periods. The low morbidity, repeatability, and lack of other options warrant consider-ation of this therapy in patients with complex fistulas.
- 3. Endoanal advancement flaps may be used for treatment of complex anal fistula disease. Grade of Rec-ommendation: Strong recommendation based on mod-erate-quality evidence 1C
Endoanal advancement flap is another sphincter-spar-ing technique that consists of curettage of the tract, and mobilizing a segment of proximal healthy anorectal mu-cosa, submucosa, and muscle to cover the site of the su-tured internal opening. In general, recurrence rates range from 13% to 56%.81– 83 The addition of fibrin glue to obliterate the tract has failed to improve success rates.81,84 Factors associated with failed repair include radiation, underlying Crohn’s disease, active proctitis, rectovaginal fistula, malignancy, and number of prior attempted re-pairs.53,82,85– 88 Although the sphincter is not divided with endoanal advancement flaps, mild or moderate inconti-nence is still reported in up to 7%–38% of patients, with associated decreases reported in both resting and squeeze pressures on postoperative manometry.85,89 –91
- 4. Complex anal fistulas may be treated by the use of a seton and/or staged fistulotomy. Grade of Recommen-dation: Strong recommendation based on moderate-quality evidence 1B
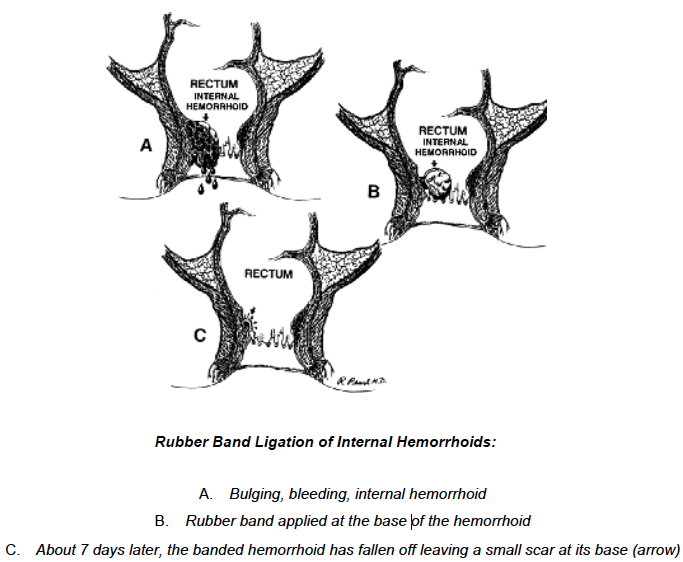
The seton (ie, suture, rubber band, Silastic vessel loop) is passed through the fistula tract to convert an inflamma-tory process to a foreign body reaction causing perisphinc-teric fibrosis. Setons may be of the cutting type, for which progressive tightening will produce a gradual fistulotomy with scarring of the tract, over the course of weeks. Alter-natively, a loose seton may be placed to promote drainage and avoidance of recurrent perineal sepsis, and may be left in place long-term or removed with ultimate cure. There remains a lack of high-quality data with regard to setons, with only 4 randomized controlled trials to date, all with varying results.69,92–94
In the setting of complex anal fistulas, setons are com-monly used in a staged fashion, with initial seton place-ment to control sepsis followed by a secondary procedure (ie, endoanal advancement flap, fibrin glue, anal plug) weeks later to avoid division of the sphincter muscle.95 Success rates in this setting have ranged from 62% to 100%, depending on the type of secondary procedure.95–99 Changes in continence range from 0% to 54% with pa-tients undergoing 2-staged procedures or cutting setons. Incontinence to flatus is seen more often than liquid or solid stool incontinence.96 –101 Finally, in sepsis secondary to fistula disease that is recalcitrant to other methods, di-version and appropriate drainage may be required.
- 5. Complex fistulas may be treated with ligation of the intersphincteric fistula tract (LIFT). Grade of Recom-mendation: No recommendation
A relatively new technique called the LIFT procedure involves ligation and division of the fistula tract in the in-tersphincteric space.102–104 The procedure typically in-volves placement of a seton for 8 or more weeks to allow fibrosis of the tract. Using an intersphincteric approach, the tract can be identified, ligated, and divided, with pos-sible closure of the internal opening and widening of the external opening for drainage. Using this approach, there is no sphincter muscle divided and, theoretically, conti-nence is preserved.
Although there are only a few small series to date in the literature, successful closure has been reported in 57% to 94% at a mean follow-up of 3 to 8 months, with a recur-rence rate of 6% to 18%.102–104 In the 3 major series to date, there have been no major changes in continence or morbidity. Unfortunately, data are too preliminary to make a formal recommendation as to their ultimate ex-pected outcomes and place in the treatment algorithm. This parameter will be updated as more evidence becomes available.
Treatment of Perianal Fistula Associated With Crohn’s Disease
Perianal pathology occurs in 40% to 80% of patients with Crohn’s disease with perianal fistula presenting a particular challenge.105 The primary treatment for perianal Crohn’s fistulas is medical; surgery is reserved for the control of sepsis and occasionally as an adjunct for cure. Antibiotics are effective, especially in fistulizing disease, with metroni-dazole and fluoroquinolones demonstrating improved perianal symptoms (at least temporarily) in over 90% of patients.106 Limited data for azathioprine, 6-mercaptopu-rine, cyclosporine, and tacrolimus have also reported some success for fistulizing Crohn’s disease.107–109 Finally, infliximab, a monoclonal antibody against tumor necrosis factor, has been shown to increase the healing rate of perianal fistulas, with complete closure in up to 46% of patients.110 The decision to embark on surgical treatment for perianal Crohn’s disease must be individualized and based on the extent of disease and the severity of symptoms. Unfortunately, despite all efforts, disease may result in proc-tectomy or permanent diversion in patients with severe perianal fistulizing disease.111–114
- 1. Asymptomatic fistulas in patients with Crohn’s disease do not require surgical treatment. Grade of Recommendation: Strong recommendation based on low-quality evidence 1C
Anal fistulas in patients with perianal Crohn’s disease may be secondary to either Crohn’s disease or cryptoglandular origin. Irrespective of etiology, patients with asymptomatic fistulas and no signs of local sepsis require no surgical intervention.115,116 These fistulas may remain dormant for an extended period of time; therefore, patients need not be subjected to the morbidity of operative intervention.
- 2. Symptomatic simple low Crohn’s fistulas may be treated by fistulotomy. Grade of Recommendation: Strong recommendation based on low-quality evidence 1C
Fistulotomy is safe and effective in low-lying simple fistulas involving no or minimal external anal sphinc-ter.106,117 Given the chronicity of the disease and high frequency of disease relapse, maximum preservation of sphincter function is essential. Thus, before embarking on any fistulotomy, surgeons should consider all relevant pa-tient factors, in particular, the extent of anorectal disease, sphincter status and continence, rectal compliance, presence of active proctitis, previous anorectal operations, and stool consistency. With proper patient selection, healing rates following fistulotomy are reported in 56% to 100% of patients, with mild incontinence rates of 6% to 12%.54,96,117–119 This may be, in part, secondary to repeated fistulotomy in patients with recurrent low fistulas. Wound healing in this patient population may be delayed by 3 to 6 months.
- 3. Complex Crohn’s fistulas may be well palliated with long-term draining setons. Grade of Recommendation: Strong recommendation based on low-quality evidence 1C
For complex fistulas associated with Crohn’s disease, long-term (�6 wk) placement of loose setons, such as vessel loops or Silastic catheters, can successfully control drainage and allow inflammation to resolve by providing continuous drainage and preventing closure of the external skin opening.105,106,117 Despite this technique, recur-rent sepsis occurs in 20% to 40%, with approximately 8%to 13% of patients experiencing some degree of fecal soil-age.8,99,120 Recent data on the use of concurrent seton drainage and infliximab therapy have reported fistula clo-sure in 24% to 78% of patients following induction therapy, with 25% to 100% of these patients responding to subsequent courses of infliximab therapy.121–123
- 4. Complex Crohn’s fistulas may be treated with advancement flap closure if the rectal mucosa is grossly normal. Grade of Recommendation: Weak recommendation based on low-quality evidence 2C
Endorectal and anodermal advancement flaps may also be used in complex Crohn’s fistulas in select patients without active proctitis. Short-term success is reported to range between 64% and 75%.82,88,124,125 Recurrence rates increase over time with extended follow-up.113,126 Recto-vaginal fistulas associated with Crohn’s have a short-term success rates of 40% to 50% when treated with a flap.124,127 Treatment with biologics to cause remission of active proctitis may permit the use of an anal flap at a later date.
- 5. Complex Crohn’s fistulas may require permanent diversion or proctectomy for uncontrollable symptoms. Grade of Recommendation: Strong recommendation based on low-quality evidence 1C
A small percentage of patients with extensive and aggressive disease that is uncontrolled by medical management and long-term seton placement may require diversion or proctectomy to control perianal sepsis.123 For patients with complex perianal Crohn’s disease, diversion rates range from 31% to 49%. Concomitant colonic disease, persistent anal sepsis, prior temporary diversion, fecal incontinence, and anal canal stenosis are reported as pre-dictive factors.112,128 Despite optimal medical and mini-mally invasive therapy, 8% to 40% will require proctec-tomy to control recalcitrant symptoms.106,113,123,129
The practice parameters set forth in this document have been developed from sources believed to be reliable. The American Society of Colon and Rectal Surgeons makes no warranty, guarantee, or representation whatsoever as to the absolute validity or sufficiency of any parameter included in this document, and the Society assumes no responsibility for the use of the material contained.
APPENDIX A: CONTRIBUTING MEMBERS OF THE ASCRS STANDARDS COMMITTEE
W. Donald Buie, M.D., Chair; Janice Rafferty, M.D., Co-chair; Farshid Araghizadeh, M.D.; Robin Boushey, M.D.; Srihdhar Chalasani, M.D.; George Chang, M.D.; Robert Cima, M.D.; Gary Dunn, M.D.; Daniel Feingold, M.D.; Phillip Fleshner, M.D.; Daniel Geisler, M.D.; Jill Jenua, M.D.; Sharon Gregorcyk, M.D.; Daniel Herzig, M.D.; An-dreas Kaiser, M.D.; Ravin Kumar, M.D.; David Larson, M.D.; Stephen Mills, M.D.; John Monson, M.D.; W. Brian Perry, M.D.; P. Terry Phang, M.D.; David Rivadeneira, M.D.; Howard Ross, M.D.; Peter Senatore, M.D.; Elin Sig-urdson, M.D.; Thomas Stahl, M.D.; Scott Steele, M.D.; Scott Strong, M.D.; Charles Ternent, M.D.; Judith Trudel, M.D.; Madhulika Varma, M.D.; Martin Weiser, M.D.
REFERENCES
1. Parks AG. Pathogenesis and treatment of fistula-in-ano. Br Med J. 1961;5224:463– 469.
2. Read DR, Abcarian H. A prospective survey of 474 patients with anorectal abscess. Dis Colon Rectum. 1979;22:566 –568.
3. Ha¨ma¨l¨ainen KP, Sainio AP. Incidence of fistulas after drainage of acute anorectal abscesses. Dis Colon Rectum. 1998;41:1357–1361.
4. Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12.
5. Parks AG, Stitz RW. The treatment of high fistula-in-ano. Dis Colon Rectum. 1976;19:487– 499.
6. Sangwan YP, Rosen L, Riether RD, Stasik JJ, Sheets JA, Khub-chandani IT. Is simple fistula-in-ano simple? Dis Colon Rec-tum. 1994;37:885– 889.
7. Kondylis PD, Shalabi A, Kondylis LA, Reilly JC. Male cryp-toglandular fistula surgery outcomes: a retrospective analysis. Am J Surg. 2009;197:325–330.
8. Galis-Rozen E, Tulchinsky H, Rosen A, et al. Long-term out-come of loose-seton for complex anal fistula: a two-centre study of patients with and without Crohn’s disease [published online ahead of print February 7, 2009]. Colorectal Dis. doi: 10.1111/j.1463-1318.2009.01796.x.
9. Whiteford MH, Kilkenny J III, Hyman N, et al.; The Standards Practice Task Force; The American Society of Colon and Rectal Surgeons. Practice parameters for the treatment of perianal abscess and fistula-in-ano (revised). Dis Colon Rectum. 2005; 48:1337–1342.
10. Schunemann HJ, Jaeschke R, Cook DJ, et al. An official ATS statement: grading the quality of evidence and strength of rec-ommendations in the ATS guidelines and recommendations. Am J Respir Crit Care Med. 2006;174:605– 614.
11. Guyatt G, Gutermen D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guide-lines: report from an American College of Chest Physicians Task Force. Chest. 2006;129:174 –181.
12. Nelson J, Billingham R. Pilonidal disease and hidradenitis sup-purativa. In: Wolff BG, Fleshman JW, Beck DE, Pemberton JH, Wexner SD, eds. The ASCRS Textbook of Colon and Rectal Sur-gery. New York: Springer; 2007:228 –235.
13. Buchmann P, Keighley MR, Allan RN, Thompson H, Alexan-der-Williams J. Natural history of perianal Crohn’s disease. Ten year follow-up: a plea for conservatism. Am J Surg. 1980; 140:642– 644.
14. Cirocco WC, Reilly JC. Challenging the predictive accuracy of Goodsall’s rule for anal fistulas. Dis Colon Rectum. 1992;35: 537–542.
15. Chinn BT. Outpatient management of pilonidal disease. Semin Colon Rectal Surg. 2003;14:166 –172.
16. Weisman RI, Orsay CP, Pearl RK, Abcarian H. The role of 17. Kuijpers HC, Schulpen T. Fistulography for fistula-in-ano: is it useful? Dis Colon Rectum. 1985;28:103–104.
18. Toyonaga T, Matsushima M, Tanaka Y, et al. Microbiological analysis and endoanal ultrasonography for diagnosis of anal fistula in acute anorectal sepsis. Int J Colorectal Dis. 2007;22: 209 –213.
19. Toyonaga T, Tanaka Y, Song JF, et al. Comparison of accuracy of physical examination and endoanal ultrasonography for preoperative assessment in patients with acute and chronic anal fistula. Tech Coloproctol. 2008;12:217–223.
20. Buchanan GN, Halligan S, Bartram CI, Williams AB, Tarroni D, Cohen CR. Clinical examination, endosonography, and MR imaging in preoperative assessment of fistula in ano: compar-ison with outcome-based reference standard. Radiology. 2004; 233:674 – 681.
21. Santoro GA, Fortling B. The advantages of volume rendering in three-dimensional endosonography of the anorectum. Dis Co-lon Rectum. 2007;50:359 –368.
22. West RL, Zimmerman DD, Dwarkasing S, et al. Prospective comparison of hydrogen peroxide-enhanced three-dimen-sional endoanal ultrasonography and endoanal magnetic reso-nance imaging of perianal fistulas. Dis Colon Rectum. 2003;46: 1407–1415.
23. Buchanan GN, Bartram CI, Williams AB, Halligan S, Cohen CR. Value of hydrogen peroxide enhancement of three-dimen-sional endoanal ultrasound in fistula-in-ano. Dis Colon Rec-tum. 2005;48:141–147.
24. West RL, Dwarkasing S, Felt-Bersma RJ, et al. Hydrogen per-oxide-enhanced three-dimensional endoanal ultrasonography and endoanal magnetic resonance imaging in evaluating peri-anal fistulas: agreement and patient preference. Eur J Gastroen-terol Hepatol. 2004;16:1319 –1324.
25. Guillaumin E, Jeffrey RB Jr, Shea WJ, Asling CW, Goldberg HI. Perirectal inflammatory disease: CT findings. Radiology. 1986; 161:153–157.
26. Yousem DM, Fishman EK, Jones B. Crohn disease: perianal and perirectal findings at CT. Radiology. 1988;167:331–334.
27. Schaefer O, Lohrmann C, Langer M. Assessment of anal fistulas with high-resolution subtraction MR-fistulography: compari-son with surgical findings. J Magn Reson Imaging. 2004;19: 91–98.
28. Maccioni F, Colaiacomo MC, Stasolla A, Manganaro L, Izzo L, Marini M. Value of MRI performed with phased-array coil in the diagnosis and pre-operative classification of perianal and anal fistulas. Radiol Med. 2002;104:58 – 67.
29. Maier AG, Funovics MA, Kreuzer SH, et al. Evaluation of peri-anal sepsis: comparison of anal endosonography and magnetic resonance imaging. J Magn Reson Imaging. 2001;14:254 –260.
30. Sahni VA, Ahmad R, Burling D. Which method is best for imaging of perianal fistula? Abdom Imaging. 2008;33:26 –30.
31. Orsoni P, Barthet M, Portier F, Panuel M, Desjeux A, Grimaud JC. Prospective comparison of endosonography, magnetic res-onance imaging and surgical findings in anorectal fistula and abscess complicating Crohn’s disease. Br J Surg. 1999;86:360 –364.
32. Gustafsson UM, Kahvecioglu B, Astro¨m G, Ahlstro¨m H, Graf
W. Endoanal ultrasound or magnetic resonance imaging for preoperative assessment of anal fistula: a comparative study. Colorectal Dis. 2001;3:189 –197.
33. Beck DE, Fazio VW, Jagelman DG, Lavery IL, Weakley FL. Catheter drainage of ischiorectal abscesses. South Med J. 1988; 81:444 – 446.
34. Vasilveski CA. Anorectal abscesses and fistulas. In: Wolff BG, Fleshman JW, Beck DE, Pemberton JH, Wexner SD, eds. The ASCRS Textbook of Colorectal Surgery. New York: Springer; 2007:192–214.
35. Cox SW, Senagore AJ, Luchtefeld MA, Mazier WP. Outcome after incision and drainage with fistulotomy for ischiorectal abscess. Am Surg. 1997;63:686 – 689.
36. Ramanujam PS, Prasad ML, Abcarian H, Tan AB. Perianal ab-scesses and fistulas: a study of 1023 patients. Dis Colon Rectum. 1984;27:593–597.
37. Onaca N, Hirshberg A, Adar R. Early reoperation for perirectal abscess: a preventable complication. Dis Colon Rectum. 2001; 44:1469 –1473.
38. Held D, Khubchandani I, Sheets J, Stasik J, Rosen L, Riether R. Management of anorectal horseshoe abscess and fistula. Dis Colon Rectum. 1986;29:793–797.
39. Rosen SA, Colquhoun P, Efron J, et al. Horseshoe abscesses and fistulas: how are we doing? Surg Innov. 2006;13:17–21.
40. Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15–19.
41. Stewart MP, Laing MR, Krukowski ZH. Treatment of acute abscesses by incision, curettage and primary suture without antibiotics: a controlled clinical trial. Br J Surg. 1985;72:66 – 67.
42. Macfie J, Harvey J. The treatment of acute superficial abscesses: a prospective clinical trial. Br J Surg. 1977;64:264 –266.
43. North JH Jr, Weber TK, Rodriguez-Bigas MA, Meropol NJ, Petrelli NJ. The management of infectious and noninfectious anorectal complications in patients with leukemia. J Am Coll Surg. 1996;183:322–328.
44. Bu¨y¨ukas¸ik Y, Ozcebe OI, Sayinalp N, et al. Perianal infections in patients with leukemia: importance of the course of neutrophil count. Dis Colon Rectum. 1998;41:81– 85.
45. Cohen JS, Paz IB, O’Donnell MR, Ellenhorn JD. Treatment of perianal infection following bone marrow transplantation. Dis Colon Rectum. 1996;39:981–985.
46. Grewal H, Guillem JG, Quan SH, Enker WE, Cohen AM. Ano-rectal disease in neutropenic leukemic patients: operative vs nonoperative management. Dis Colon Rectum. 1994;37:1095–1099.
47. Albright JB, Pidala MJ, Cali JR, Snyder MJ, Voloyiannis T, Bailey HR. MRSA-related perianal abscesses: an underrecognized disease entity. Dis Colon Rectum. 2007;50:996 –1003.
48. Goldberg GS, Orkin BA, Smith LE. Microbiology of human immunodeficiency virus anorectal disease. Dis Colon Rectum. 1994;37:439 – 443.
49. Wilson W, Taubert KA, Gewitz T, et al. Prevention of Infective Endocarditis. Guidelines From the American Heart Associa-tion: A Guideline From the American Heart Association Rheu-matic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007; 116:1736 –1754.
50. Gonzalez-Ruiz C, Kaiser AM, Vukasin P, Beart RW Jr, Ortega AE. Intraoperative physical diagnosis in the management of anal fistula. Am Surg. 2006;72:11–15.
51. Gunawardhana PA, Deen KI. Comparison of hydrogen peroxide instillation with Goodsall’s rule for fistula-in-ano. ANZ J Surg. 2001;71:472– 474.
52. Coremans G, Dockx S, Wyndaele J, Hendrickx A. Do anal fis-tulas in Crohn’s disease behave differently and defy Goodsall’s rule more frequently than fistulas that are cryptoglandular in origin? Am J Gastroenterol. 2003;98:2732–2735.
53. Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Mad-off RD. Anal fistula surgery: factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39:723–729.
54. Davies M, Harris D, Lohana P, et al. The surgical management of fistula-in-ano in a specialist colorectal unit. Int J Colorectal Dis. 2008;23:833– 838.
55. Nwaejike N, Gilliland R. Surgery for fistula-in-ano: an audit of practice of colorectal and general surgeons. Colorectal Dis. 2007;9:749 –753.
56. Jorda´n J, Roig JV, García-Armengol J, García-Granero E, Solana A, Lledo´ S. Risk factors for recurrence and incontinence after anal fistula surgery [published online ahead of print February 7, 2009]. Colorectal Dis. doi: 10.1111/j.1463-1318.2009.01806.x.
57. van Koperen PJ, Wind J, Bemelman WA, Bakx R, Reitsma JB, Slors JF. Long-term functional outcome and risk factors for recurrence after surgical treatment for low and high perianal fistulas of cryptoglandular origin. Dis Colon Rectum. 2008;51: 1475–1481.
58. van Tets WF, Kuijpers HC. Continence disorders after anal fistulotomy. Dis Colon Rectum. 1994;37:1194 –1197.
59. Pescatori M, Ayabaca SM, Cafaro D, Iannello A, Magrini S. Marsupialization of fistulotomy and fistulectomy wounds improves healing and decreases bleeding: a randomized controlled trial. Colorectal Dis. 2006;8:11–14.
60. Ho YH, Tan M, Leong AF, Seow-Choen F. Marsupialization of fistulotomy wounds improves healing: a randomized controlled trial. Br J Surg. 1998;85:105–107.
61. Kronborg O. To lay open or excise a fistula-in-ano: a randomized trial. Br J Surg. 1985;72:970.
62. Belmonte Montes C, Ruiz Galindo GH, Montes Villalobos JL, Decanini Tera´n C. Fistulotomy vs fistulectomy: ultrasono graphic evaluation of lesion of the anal sphincter function [in Spanish]. Rev Gastroenterol Mex. 1999;64:167–170.
63. Schouten WR, van Vroonhoven TJ. Treatment of anorectal abscess with or without primary fistulectomy: results of a prospective randomized trial. Dis Colon Rectum. 1991;34:60 – 63.
64. Quah HM, Tang CL, Eu KW, Chan SY, Samuel M. Meta-anal-ysis of randomized clinical trials comparing drainage alone vs primary sphincter-cutting procedures for anorectal abscess-fistula. Int J Colorectal Dis. 2006;21:602– 609.
65. Adams T, Yang J, Kondylis LA, Kondylis PD. Long-term outlook after successful fibrin glue ablation of cryptoglandular transsphincteric fistula-in-ano. Dis Colon Rectum. 200;51: 1488 –1490.
66. Sentovich SM. Fibrin glue for anal fistulas: long-term results. Dis Colon Rectum. 2003;46:498 – 450.
67. Swinscoe MT, Ventakasubramaniam AK, Jayne DG. Fibrin glue for fistula-in-ano: the evidence reviewed. Tech Coloproc-tol. 2005;9:89 –94.
68. Yeung JM, Simpson JA, Tang SW, Armitage NC, Maxwell-Armstrong C. Fibrin glue for the treatment of fistulae-in-ano: a method worth sticking to? [published online ahead of print February 7, 2009] Colorectal Dis. doi: 10.1111/j.1463-1318. 2009.01801.x.
69. Lindsey I, Smilgin-Humphreys MM, Cunningham C, Mortensen NJ, George BD. A randomized, controlled trial of fibrin glue vs conventional treatment for anal fistula. Dis Colon Rectum. 2002; 45:1608–1615.
70. Loungnarath R, Dietz DW, Mutch MG, Birnbaum EH, Kodner IJ, Fleshman JW. Fibrin glue treatment of complex anal fistulas has low success rate. Dis Colon Rectum. 2004;47:432– 436.
71. de Parades V, Far HS, Etienney I, Zeitoun JD, Atienza P, Bauer
P. Seton drainage and fibrin glue injection for complex anal fistulas[published online ahead of print February 7, 2009]. Colorectal Dis. doi: 10.1111/j.1463-1318.2009.01811.x
72. Song WL, Wang ZJ, Zheng Y, Yang XQ, Peng YP. An anorectal fistula treatment with acellular extracellular matrix: a new technique. World J Gastroenterol. 2008;14:4791– 4794.
73. Ky AJ, Sylla P, Steinhagen R, Steinhagen E, Khaitov S, Ly EK. Collagen fistula plug for the treatment of anal fistulas. Dis Co-lon Rectum. 2008;51:838 – 843.
74. Ellis CN, Rostas JW, Greiner FG. Long-term outcomes with the use of bioprosthetic plugs for the management of complex anal fistulas. Dis Colon Rectum. 2010;53:798 – 802.
75. Zubaidi A, AL-Obeed O. Anal fistula plug in high fistula-in-ano: an early Saudi experience. Dis Colon Rectum. 2009;52: 1584 –1588.
76. O’Connor L, Champagne BJ, Ferguson MA, Orangio GR, Schertzer ME, Armstrong DN. Efficacy of anal fistula plug in closure of Crohn’s anorectal fistulas. Dis Colon Rectum. 2006; 49:1569 –1573.
77. Champagne BJ, O’Connor LM, Ferguson M, Orangio GR, Schertzer ME, Armstrong DN. Efficacy of anal fistula plug in closure of cryptoglandular fistulas: long-term follow-up. Dis Colon Rectum. 2006;49:1817–1821.
78. Schwandner O, Stadler F, Dietl O, Wirsching RP, Fuerst A. Initial experience on efficacy in closure of cryptoglandular and Crohn’s transsphincteric fistulas by the use of the anal fistula plug. Int J Colorectal Dis. 2008;23:319 –324.
79. Safar B, Jobanputra S, Sands D, Weiss EG, Nogueras JJ, Wexner SD. Anal fistula plug: initial experience and outcomes. Dis Co-lon Rectum. 2009;52:248 –252.
80. Christoforidis D, Etzioni DA, Goldberg SM, Madoff RD, Mell-gren A. Treatment of complex anal fistulas with the collagen fistula plug. Dis Colon Rectum. 2008;51:1482–1487.
81. van Koperen PJ, Wind J, Bemelman WA, Slors JF. Fibrin glue and transanal rectal advancement flap for high transsphinc-teric perianal fistulas: is there any advantage? Int J Colorectal Dis. 2008;23:697–701.
82. Mizrahi N, Wexner SD, Zmora O, et al. Endorectal advance-ment flap: are there predictors of failure? Dis Colon Rectum. 2002;45:1616 –1621.
83. Mitalas LE, Gosselink MP, Zimmerman DD, Schouten WR. Repeat transanal advancement flap repair: impact on the over-all healing rate of high transsphincteric fistulas and on fecal continence. Dis Colon Rectum. 2007;50:1508 –1511.
84. Ellis CN, Clark S. Fibrin glue as an adjunct to flap repair of anal fistulas: a randomized, controlled study. Dis Colon Rectum. 2006;49:1736 –1740.
85. Schouten WR, Zimmerman DD, Briel JW. Transanal advance-ment flap repair of transsphincteric fistulas. Dis Colon Rectum. 1999;42:1419 –1422.
86. Zimmerman DD, Briel JW, Gosselink MP, Schouten WR. Ano-cutaneous advancement flap repair of transsphincteric fistulas. Dis Colon Rectum. 2001;44:1474 –1480.
87. Jones IT, Fazio VW, Jagelman DG. The use of transanal rectal advancement flaps in the management of fistulas involving the anorectum. Dis Colon Rectum. 1987;30:919 –923.
88. Sonoda T, Hull T, Piedmonte MR, Fazio VW. Outcomes of primary repair of anorectal and rectovaginal fistulas using the endorectal advancement flap. Dis Colon Rectum. 2002;45: 1622–1628.
89. Athanasiadis S, Helmes C, Yazigi R, Ko¨hler A. The direct clo-sure of the internal fistula opening without advancement flap for transsphincteric fistulas-in-ano. Dis Colon Rectum. 2004; 47:1174 –1180.
90. Perez F, Arroyo A, Serrano P, Sa´nchez A, et al. Randomized clinical and manometric study of advancement flap versus fis-tulotomy with sphincter reconstruction in the management of complex fistula-in-ano. Am J Surg. 2006;192:34 – 40.
91. Uribe N, Milla´n M, Minguez M, et al. Clinical and manometric results of endorectal advancement flaps for complex anal fis-tula. Int J Colorectal Dis. 2007;22:259 –264.
92. Zbar AP, Ramesh J, Beer-Gabel M, Salazar R, Pescatori M. Conventional cutting vs. internal anal sphincter-preserving se-ton for high trans-sphincteric fistula: a prospective random-ized manometric and clinical trial. Tech Coloproctol. 2003;7: 89 –94.
93. Shukla N. Multicentric randomized controlled clinical trial of Kshaarasootra (Ayurvedic medicated thread) in the manage-ment of fistula-in-ano. Indian Council of Medical Research. Indian J Med Res. 1991;94:177–185.
94. Ho KS, Tsang C, Seow-Choen F, et al. Prospective randomised trial comparing ayurvedic cutting seton and fistulotomy for low fistula-in-ano. Tech Coloproctol. 2001;5:137–141.
95. Tyler KM, Aarons CB, Sentovich SM. Successful sphincter-sparing surgery for all anal fistulas. Dis Colon Rectum. 2007;50: 1535–1539.
96. Williams JG, MacLeod CA, Rothenberger DA, Goldberg SM. Seton treatment of high anal fistulae. Br J Surg. 1991;78:1159 –1161.
97. Isbister WH, Al Sanea N. The cutting seton: an experience at King Faisal Specialist Hospital. Dis Colon Rectum. 2001;44: 722–727.
98. Mentes BB, Oktemer S, Tezcaner T, Azili C, Leventog˘lu S, Og˘uz
M. Elastic one-stage cutting seton for the treatment of high anal fistulas: preliminary results. Tech Coloproctol. 2004;8:159 –162.
99. Eitan A, Koliada M, Bickel A. The use of the loose seton tech-nique as a definitive treatment for recurrent and persistent high trans-sphincteric anal fistulas: a long-term outcome [pub-lished online ahead of print February 24, 2009]. J Gastrointest Surg. doi: 10.1007/s11605-009-0826-6.
100. Theerapol A, So BY, Ngoi SS. Routine use of setons for the treatment of anal fistulae. Singapore Med J. 2002;43:305–307.
101. Chuang-Wei C, Chang-Chieh W, Cheng-Wen H, Tsai-Yu L, Chun-Che F, Shu-Wen J. Cutting seton for complex anal fistu-las. Surgeon. 2008;6:185–188.
102. Bleier JL, Moloo H, Goldberg SM. Ligation of the intersphinc-teric fistula tract: an effective new technique for complex fistu-las. Dis Colon Rectum. 2010;53:43– 46.
103. Shanwani A, Nor AM, Amri N. Ligation of the intersphincteric fistula tract (LIFT): a sphincter-saving technique for fistula-in-ano. Dis Colon Rectum. 2010;53:39 – 42.
104. Rojanasakul A, Pattanaarun J, Sahakitrungruang C, Tan-tiphlachiva K. Total anal sphincter saving technique for fistula-in-ano: the ligation of the intersphincteric fistula tract. J Med Assoc Thai. 2007;90:581–586.
105. Makowiec F, Jehle EC, Becker HD, Starlinger M. Perianal ab-scess in Crohn’s disease. Dis Colon Rectum. 1997;40:443– 450.
106. McKee RF, Keenan RA. Perianal Crohn’s disease: is it all bad news? Dis Colon Rectum. 1996;39:136 –142.
107. Korelitz BI, Present DH. Favorable effect of 6-mercaptopurine on fistulae of Crohn’s disease. Dig Dis Sci. 1985;30:58 – 64.
108. Sandborn WJ, Present DH, Isaacs KL, et al. Tacrolimus for the treatment of fistulas in patients with Crohn’s disease: a ran-domized, placebo-controlled trial. Gastroenterology. 2003;125: 380 –388.
109. Present DH, Lichtiger S. Efficacy of cyclosporine in treatment of fistula of Crohn’s disease. Dig Dis Sci. 1994;39:374 –380.
110. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398 –1405.
111. Yamamoto T, Allan RN, Keighley MR. Effect of fecal diversion alone on perianal Crohn’s disease. World J Surg. 2000;24:1258 –1262.
112. Galandiuk S, Kimberling J, Al-Mishlab TG, Stromberg AJ. Perianal Crohn disease: predictors of need for permanent diversion. Ann Surg. 2005;241:796 – 801.
113. Lo¨ffler T, Welsch T, Mu¨hl S, Hinz U, Schmidt J, Kienle P. Long-term success rate after surgical treatment of anorectal and rectovaginal fistulas in Crohn’s disease [published online ahead of print January 27, 2009]. Int J Colorectal Dis. doi: 10.1007/s00384-009-0638-x.
114. Gaertner WB, Decanini A, Mellgren A, et al. Does infliximab infusion impact results of operative treatment for Crohn’s perianal fistulas? Dis Colon Rectum. 2007;50:1754 –1760.
115. Solomon MJ. Fistulae and abscesses in symptomatic perineal Crohn’s disease. Int J Colorectal Dis. 1996;11:222–226.
116. Mardini HE, Schwartz DA. Treatment of perianal fistula and abscess: Crohn’s and non-Crohn’s. Curr Treat Options Gastro-enterol. 2007;10:211–220.
117. Sangwan YP, Schoetz DJ Jr, Murray JJ, et al. Perianal Crohn’s disease: results of local surgical treatment. Dis Colon Rectum. 1996;39:529 –535.
118. Williamson PR, Hellinger MD, Larach SW, Ferrara A. Twenty year review of the surgical management of perianal Crohn’s disease. Dis Colon Rectum. 1995;38:389 –392.
119. Pescatori M, Interisano A, Basso L, et al. Management of perianal Crohn’s disease: results of a multicenter study in Italy. Dis Colon Rectum. 1995;38:121–124.
120. Takesue Y, Ohge H, Yokoyama T, Murakami Y, Imamura Y, Sueda T. Long-term results of seton drainage on complex anal fistulae in patients with Crohn’s disease. J Gastroenterol. 2002; 37:912–915.
121. Topstad DR, Panaccione R, Heine JA, Johnson DR, MacLean AR, Buie WD. Combined seton placement, infliximab infusion, and maintenance immunosuppressives improve healing rate in fistulizing anorectal Crohn’s disease: a single center experience. Dis Colon Rectum. 2003;46:577–583.
122. Guidi L, Ratto C, Semeraro S, et al. Combined therapy with infliximab and seton drainage for perianal fistulizing Crohn’s disease with anal endosonographic monitoring: a single centre experience. Tech Coloproctol. 2008;12:1111–1117.
123. Hyder SA, Travis SP, Jewell DP, McC Mortensen NJ, George BD. Fistulating anal Crohn’s disease: results of combined surgical and infliximab treatment. Dis Colon Rectum. 2006;49: 1837–1841.
124. Hull TL, Fazio VW. Surgical approaches to low anovaginal fistula in Crohn’s disease. Am J Surg. 1997;173:95–98.
125. Joo JS, Weiss EG, Nogueras JJ, Wexner SD. Endorectal advancement flap in perianal Crohn’s disease. Am Surg. 1998;64: 147–150.
126. Ozuner G, Hull TL, Cartmill J, Fazio VW. Long-term analysis of the use of transanal rectal advancement flaps for complicated anorectal/vaginal fistulas. Dis Colon Rectum. 1996;39: 10 –14.
127. Hyman N. Endoanal advancement flap repair for complex anorectal fistulas. Am J Surg. 1999;178:337–340.
128. Mueller MH, Geis M, Glatzle J, et al. Risk of fecal diversion in complicated perianal Crohn’s disease. J Gastrointest Surg. 2007; 11:529 –537.
129. Bell SJ, Williams AB, Wiesel P, Wilkinson K, Cohen RC, Kamm MA. The clinical course of fistulating Crohn’s disease. Aliment Pharmacol Ther. 2003;17:1145–1151.