Practice Parameters for Anal Squamous Neoplasms
Scott R. Steele, M.D., Madhulika G. Varma, M.D., Genevieve B. Melton, M.D.,
Howard M. Ross, M.D., Janice F. Rafferty, M.D., W. Donald Buie, M.D., on behalf of the Standards Practice Task Force of the American Society of Colon and Rectal Surgeons
The American Society of Colon and Rectal Surgeons is dedicated to ensuring high-quality patient care by advancing the science, prevention, and management of disorders and diseases of the colon, rectum, and anus. The Standards Committee is composed of Society members who are chosen because they have demonstrated expertise in the specialty of colon and rectal surgery. This Committee was created to lead international efforts in defining quality care for conditions related to the colon, rectum, and anus. This is accompanied by developing Clinical Practice Guidelines based on the best available evidence. These guidelines are inclusive and not prescriptive. Their purpose is to provide information on which decisions can be made, rather than dictate a specific form of treatment. These guidelines are intended for the use of all practitioners, health care workers, and patients who desire information about the management of the conditions addressed by the topics covered in these guidelines.
It should be recognized that these guidelines should not be deemed inclusive of all proper methods of care or exclusive of methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding the propriety of any specific procedure must be made by the physician in light of all the circumstances presented by the individual patient.
Statement Of The Problem
Squamous-cell carcinoma (SCC) of the anus remains a relatively rare malignancy, with the American Cancer Society estimating approximately 5260 new cases (3260 in women; 2000 in men) and 720 deaths in the United States for 2010 alone.1 Although accounting for only ~2% of all colorectal malignancies, the incidence continues to steadily rise, up from ~4600 cases in 2006.2 Anal cancer has several histological variants, including cloacogenic, basaloid, epidermoid, and mucoepidermoid carcinomas, with squamous cell comprising the vast majority.3 Because of their similar presentation, response to treatment, and eventual outcome, anal cancers are often grouped together.4 Large population-based data suggest that approximately half of patients present with localized disease, one-third with regional nodal disease, and 10% to 15% with distant metastases.5,6 Epidemiologic studies have also demonstrated a strong, putatively causal, relationship with infection with the human papillomavirus (HPV), particularly serotypes 16 and 18.7 Although the overall relative infrequency of this disease has historically led to a limited number of adequately powered studies on which to base high-grade recommendations for diagnosis and treatment, this emerging epidemic allows sufficient data upon which to develop evidence-based clinical practice guidelines. In addition, there has been an evolution of not only the terminology of these lesions, but also particular attention to strict location definitions to provide a more uniform assessment of outcomes.
Anal intraepithelial neoplasia (AIN) is a precursor lesion to anal SCC and is further stratified into 3 grades: AIN I, AIN II , and AIN III , which indicate low-, moderate-, and high-grade dysplasia, and correspond to the histological findings, including cytologic changes, mitotic activity, nuclear membrane changes, and abnormalities in squamous-cell maturation/differentiation.8,9 Bowen disease is synonymous with carcinoma in situ, but, as a term, it should be avoided to decrease confusion. Similarly, the terms high-grade squamous intraepithelial lesion (HSIL) and low-grade squamous intraepithelial lesion (LSIL) have been suggested. In this classification, the term LSIL corresponds to AIN I, and HSIL encompasses AIN II and AIN III (carcinoma in situ and Bowen disease).10 Because these terms are more appropriately reserved for the cytologic abnormalities rather than the true histology, more recently the terms high-grade (HGAIN) and low-grade (LGAIN) anal intraepithelial neoplasia have been proposed that correspond to AIN IIIIIIIII and AIN I/IIII, respectively. Although terminology in the perianal region continues to evolve over time, this practice parameter will use the terms HGAIN and LGAIN, which, like anal cancer, has also demonstrated a similar increase in incidence, especially in immunosuppressed
patients.6,11–16 Although various tumors can arise in the anal canal and perianal region, this practice parameter will focus strictly on LGAIN/HGAIN and squamous-cell anal neoplasms. For the purposes of this parameter, anal canal cancers are defined as tumors that originate from mucosa of the anus, in contrast to those that arise within skin or distal to the mucocutaneous junction that are termed anal margin tumors. Anatomically, per American Joint Commission on Cancer (AJCC) definitions, the anal canal “begins where the rectum enters the puborectalis sling at the apex of the anal sphincter complex (palpable as the anorectal ring on digital examination and approximately 1–2 cm proximal to the dentate line) and ends at the squamous mucosa blending with the perianal skin.”17 Anal margin tumors’ boundaries are indistinct, but generally extend radially 5 to 6 cm from the squamous mucocutaneous junction, although this may vary slightly based on individual body habitus.1
Methodology
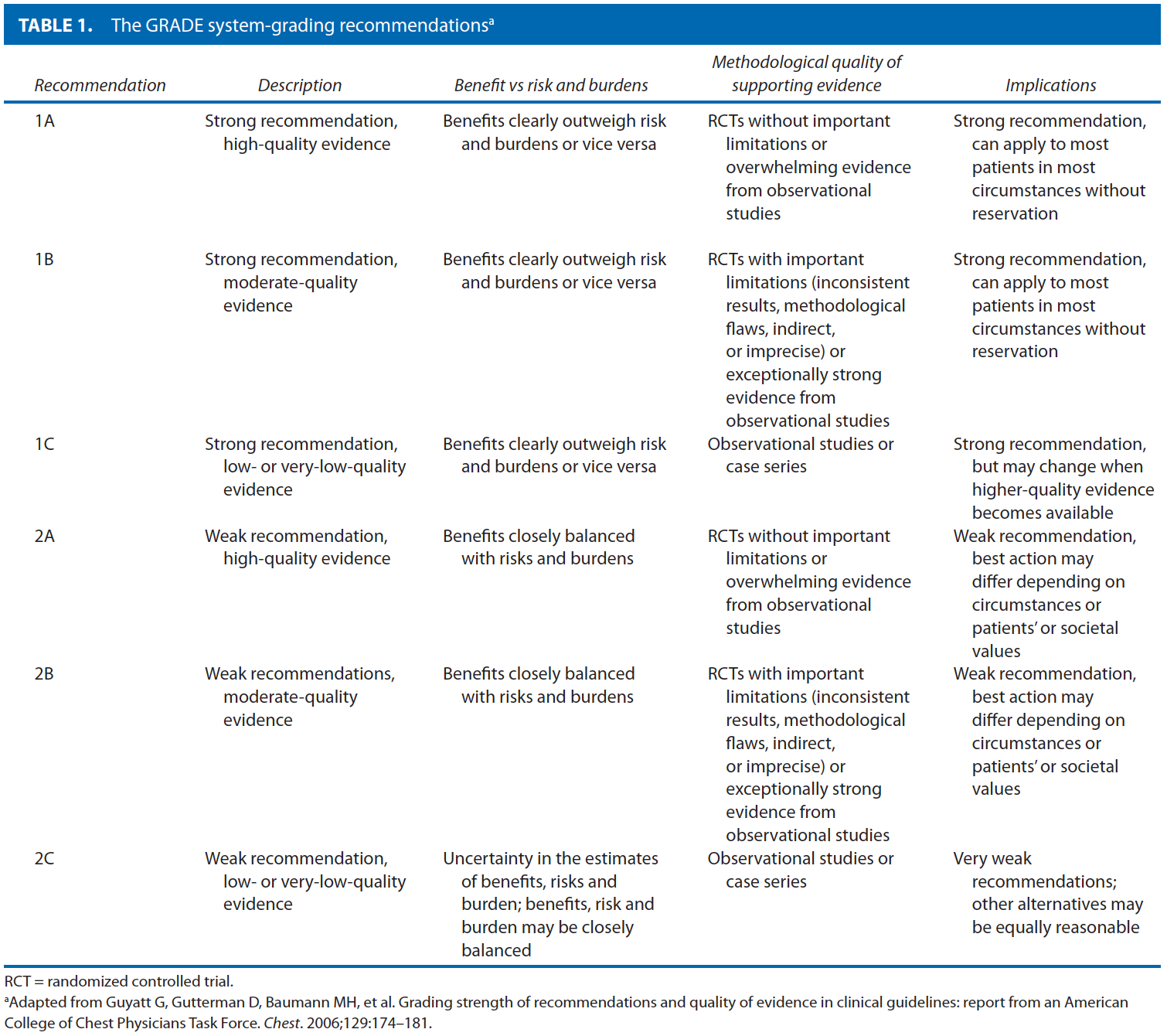
These guidelines are built on the last set of the American Society of Colon and Rectal Surgeons Practice Parameters for treatment of anal squamous neoplasms published in 2008.18 An updated organized search of MEDLINE, PubMed, Embase, and the Cochrane Database of Collected Reviews was performed through June 2011. Key-word combinations included “AIN,” “anal cancer,” “anal malignancy,” “anal carcinoma,” “anal intraepithelial neoplasia,” “Bowen’s disease,” “anal margin,” “anal canal,” “LSIL,’; “HSIL,” “Nigro protocol,” “HPV,” “human papilloma virus,” “vaccination,” “anal surgery,” “chemotherapy,” “radiation therapy,” and “squamous-cell cancer.” Directed searches of the embedded references from the primary articles were also performed in selected circumstances. Although not exclusionary, primary authors focused on all English language articles and studies of adults. Recommendations were formulated by the primary authors and reviewed by the entire Standards Committee. The final grade of recommendation was performed with the use of the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) system (Table 1).19
Anal Canal Squamous-cell Carcinoma
Pretreatment Evaluation
- A. A disease-specific history should be performed, emphasizing symptoms, risk factors, and signs of advanced disease. Grade of Recommendation: Strong recommendation based on low-quality evidence, 1C.
Most patients present with a slow-growing mass located in the intraanal or perianal position.20 Pain and bleeding are common, occurring in approximately half of patients, although up to 20% of patients may be asymptomatic.21,22 Diagnosis of SCC may often be delayed, because the nonspecific symptoms are initially attributed to other benign anorectal pathology such as hemorrhoids in 70% to 80% of patients.20,23 With lymphatic spread primarily to the inguinal region, groin pain may indicate regional involvement. Risk factors associated with anal SCC include infection with the HPV, HIV seropositivity, history of other HPV-related genital malignancies (cervical cancer, cervical intraepithelial neoplasia (CIN), vulvar cancer, or vulvar intraepithelial neoplasia)13,14; previous sexually acquired diseases, cigarette smoking, anoreceptive intercourse, multiple sexual partners, history of solid organ transplant, and other forms of immunosuppression.14,16,24–28 Because the incidence of anal cancer is higher in men practicing anoreceptive intercourse with men and those with HIV positivity, a high index of suspicion should be maintained in these patients presenting with anorectal complaints.29 Although potentially sensitive and difficult, inquiry to establish the presence or absence of these risk factors is important. Certain factors such as previous radiation, general medical issues, or inadequately controlled HIV may prove to be limiting or contraindications to chemoradiation therapy (CRT) or radical surgery, and are important to determine at the time of diagnosis.
-
B. A disease-specific physical examination should be performed to determine size, possible lymph node involvement, or metastatic disease. Grade of Recommendation: Strong recommendation based on moderate-quality evidence, 1B.
Physical examination should focus primarily on the anorectal examination and evaluation of the inguinal and femoral nodes.30 Digital rectal examination should be performed to identify the lesion location and to evaluate for fixation and the presence of sphincter invasion. Perirectal lymph nodes may be sometimes palpable. Anoscopy or proctoscopy with biopsy is essential to establish the size of the lesion, to determine the location within the anal canal, and to confirm diagnosis. Presence of palpable inguinal lymphadenopathy can suggest the need for fine-needle aspiration or core biopsy to confirm malignant involvement and help guide radiation fields. In general, metastatic disease is difficult to detect on physical examination, although a complete physical examination should be performed to help identify any potential sites of distant spread that may warrant further evaluation.
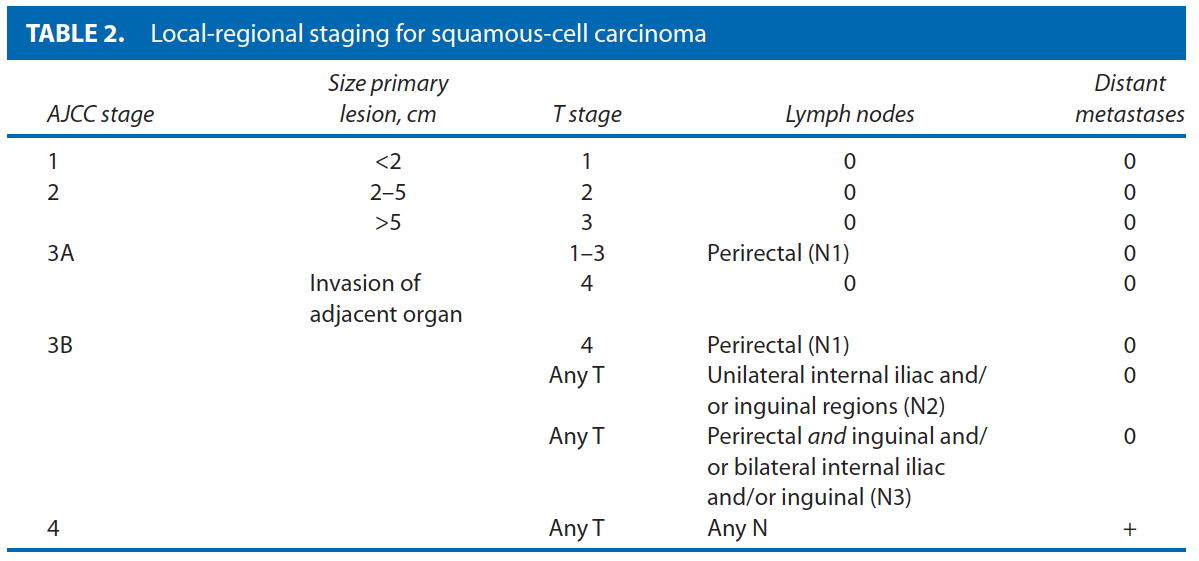
The AJCC17 local-regional staging for SCC of the anal canal focuses on the primary lesion size, the existence of local invasion, and the presence or absence of regional node disease. As such, clinical evaluation including size is critically important to determine proper staging (Table 2 ). Invasion of the anal sphincter or perianal skin does not constitute a T4 lesion; however, this should be determined to aid in potential alterations in treatment.31
-
C. Endoscopic and radiologic evaluation should be performed to help determine staging, and concomitant or metastatic disease. Grade of Recommendation: Strong recommendation based on moderate-quality evidence, 1B.
Biopsy should be performed under direct vision or through an anoscope or sigmoidoscope. Although anal cancer is not a risk factor for the development of colon cancer, colorectal neoplasms have been demonstrated in up to 15% of patients with anal cancer; therefore, colonoscopy should be performed to rule out synchronous colorectal neoplasms based on standard age and risk profile assessment.32,33 Chest, abdomen, and pelvic CT should be performed to evaluate for lymphadenopathy, in particular, inguinal lymph node radiographic abnormalities that may warrant biopsy, and to exclude lung and liver metastases.4 Because SCC can metastasize not infrequently to the brain, head CT may be performed if clinical symptoms suggest involvement. In addition, evaluation of the primary tumor may be considered. Endoanal ultrasound (EAUS) and MRI are presently the 2 most accepted modalities and may be useful in determining primary tumor depth, evaluating sphincter involvement, and evaluating perirectal lymph node involvement, with data reporting increased accuracy and sensitivity over physical examination alone.34,35 MRI is comparable to EAUS for primary tumor size and nodal status and may be considered.36 Although not typically a part of the routine evaluation, FDG-PET/CT has been shown to identify distant metastases that are not detected by physical examination or other imaging modalities in 17% to 25%,37,38 resulting in a reported change in treatment (ie, radiotherapy) in up to 5% to 19% of cases.39–41
- D. Sentinel lymph node evaluation for detection of regional nodal metastases is still investigational. Grade of Recommendation: Weak recommendation based on low-quality evidence, 2C.
Small case series have identified sentinel lymph node (SLN) evaluation of inguinal nodes to be superior to physical examination, CT, and PET/CT for the detection and staging of regional nodes.42,43 Both indocyanine green and technetium-(99)m sulfur colloid injection along with blue dye methods have been described with successful results in identifying nodal metastases.44 Overall detection rates of SLN have ranged from 73% to 100%.44,45 There are a few reports of patients with false-negative SLN not receiving radiation therapy and subsequent development of nodal disease, although the false-negative rate is generally low.45 Although clearly technically feasible at present, the exact role of SLN evaluation remains to be determined.
Treatment
Primary Treatment
- 1. The primary treatment for most SCCs of the anal canal should be combined modality CRT. Grade of Recommendation: Strong recommendation based on high-quality evidence, 1A.
Historically, anal canal cancer was treated by abdominoperineal resection (APR). However, since Nigro’s demonstration of complete tumor response with equivalent rates of disease-free and overall survival along with added benefit of sphincter preservation, combined modality CRT has become the primary treatment for most patients.46,47 The radiation fields typically include the primary tumor bed and the inguinal nodal basins. Unfortunately, despite the improved outcomes, national cancer registry data have shown ~25% of patients did not receive primary CRT as recently as 2003 to 2005.48 Whereas Nigro’s protocol originally consisted of 5-fluorouracil (5-FU), mitomycin C (MMC), and 3000 cGy of external beam radiation to the pelvis, several different treatment regimens have followed.49,50 Multiple prospective, multi-institutional, randomized controlled trials have demonstrated benefits including lower rates of local failure, tumor recurrence, and need for colostomy when using combined CRT over radiation therapy alone for the first-line therapy of anal cancer.51–55 Although there seems to be no significant change in overall survival, a higher disease-free survival has been reported with combined CRT. Combined therapy, however, has been associated with an increased incidence of hematological toxicity.56 Radiation therapy alone may be considered in patients who cannot tolerate the additional toxicity of chemotherapy.56,57 Local excision is an appropriate consideration only for small superficial lesions outside the anal canal at the anal margin in most instances.58 For those early lesions with node-negative disease and close margins (<1 mm) or residual microscopic disease on biopsy, consideration may be given to additional low-dose, reduced-volume CRT.59
- 1b. Intensity-modulated radiation therapy-based chemoradiotherapy (IMRT) may be considered to decrease treatment-related toxicity. Grade of Recommendation: Weak recommendation based on moderate-quality evidence, 2B.
One of the drawbacks to CRT, especially with the more recent use of dose-escalating radiation is the treatment-related side effects and resulting breaks in therapy—occurring in up to 40% to 60% of patients.60–62 IMRT involves delivery of radiation in a manner that still provides total radiation doses of up to 60 Gy for the primary lesion, while tailoring the high-dose radiation to individual tumors to minimize side effects. With the use of this regimen, all grades of radiation-induced, especially acute, toxicity (ie, hematological, skin, diarrhea) occur at significantly lower rates, while decreasing breaks in treatment to18% to 42% lasting on average 4 to 5 days.62,63 Complete primary tumor response with IMRT occurs in more than 90% of patients.62 Overall survival, local-regional survival, and colostomy-free survival rates are similar to rates of the use of traditional radiation therapy methods.63 This technique typically involves a longer treatment time, increased monitoring requirement, and an overall increase in the volume of healthy tissue exposed to radiation. In addition, IMRT has an as-yet undetermined potential risk of future secondary malignancies, and the long-term toxicities are still undetermined.64,65
- 2. Multidrug chemotherapy including MCC and 5-FU along with radiation is usually preferable to other chemotherapy regimens with radiation. Grade of Recommendation: Strong recommendation based on high-quality evidence, 1A.
Although modifications to Nigro’s original protocol have been made trying to identify the ideal regimen, the combination of MMC with 5-FU along with radiation remains the most common combination.51,54,66 A recent multicenter randomized controlled trial (RTOG 98-11) compared 5-FU/MMC with radiation versus induction chemotherapy with 5-FU/cisplatin followed by 5-FU/cisplatin with radiation and demonstrated similar rates of 5-year disease-free survival (60% vs 70%, p = 0.17), overall survival (75% vs 70%, p = 0.10). However, the MMC-based arm without induction chemotherapy was associated with a lower 5-year local-regional recurrence (25% vs 33%) and distant metastasis rates (15% vs 19%) in comparison with cisplatin-based therapy.67 This study has been criticized because induction chemotherapy was only given in the 5-FU/cisplatin arm, and thus the treatment strategies were not equivalent. However, based on these results, MMC/
5-FU-based chemoradiation remains a common first line for anal squamous carcinoma. MMC was also associated with lower rates of colostomy (10% vs 19%, p = 0.02) at the cost of higher rates of hematological toxicity. Secondary analysis from this US Intergroup trial identified large tumor size (>5 cm) as the only pretreatment variable predictive for colostomy need.68 More recently, the ACT II phase III study randomly assigned patients to 5-FU/MMC vs 5-FU/cisplatin.69 This study demonstrated similar rates of disease control and survival, colostomy, and overall toxicity between the 2 treatment arms. Furthermore, the 5-FU/MMC arm was still associated with a 60% rate of severe hematological toxicity.
Recent single-institutional data with median follow-up of 83 months reported similar overall and disease-free survival and colostomy-free rates with 5-FU/MMC with 5-FU/cisplatin therapy.70 Combined 5-FU/cisplatin may also be beneficial as an induction regimen for patients with poor-prognosis SCC (T3/T4 and/or N2/N3 disease).71
Additional phase II data from European trials have demonstrated a small beneficial effect of MMC combined with cisplatin and radiotherapy over 5-FU/MMC for locally advanced disease (>4 cm N0 or node positive), although overall toxicity profiles were generally worse in the MMC/cisplatin group.72,73
- 3. Higher doses of radiation therapy without prolonged breaks in treatment are preferable when tolerated. Grade of Recommendation: Weak recommendation based on moderate-quality evidence, 2B.
Ideally, an uninterrupted treatment regimen is preferred for both patient convenience and improved outcomes. Longer total treatment duration has been associated with higher rates of local regional failure (HR = 1.96) and colostomy rates (HR = 1.57) in pooled multi-institutional and retrospective data,74–76 although other reports have not found a consistent correlation between overall treatment time and local failure.77 In some instances, grade 3 and higher toxicities will result in a necessary treatment break for recovery. In contrast to treatment breaks, split-course therapy has a planned hiatus in therapy. Limiting treatment breaks has demonstrated similar local control and colostomy-free survival rates in comparison with interrupted treatment cycles.78 Others have reported worse disease-free survival with prolonged (>7day) breaks in treatment.79 The increased utilization of IMRT-based CRT regimens may ultimately decrease these treatment-related toxicities while maintaining similar outcomes.80
Higher radiation boost doses to the primary tumor to yield of 56 to 60 GY and up to 65 GY for T3/T4 lesions along with concurrent chemotherapy have demonstrated improved locoregional control in single-institution retrospective series.81 Additional doses after the completion of initial therapy do not appear to affect locoregional control and may increase the overall incidence of late morbidity,77 although limited data suggest that boost dosing with brachytherapy may be superior to external beam radiotherapy in select patients.75
Treatment of Recurrent or Persistent Disease
- 1. Abdominoperineal resection is effective salvage therapy for persistent or recurrent disease: Grade of Recommendation: Strong recommendation based on moderate-quality evidence, 1B.
Unfortunately, ~20% to 30% of patients will have persistent or recurrent disease following primary CRT.82 Predictors of recurrent/persistent locoregional disease after definitive CRT include higher T and N stage at original presentation, HIV-positive status, and the inability to complete CRT.82,83 Patients with persistent disease (present within 6 months of completing CRT) have a poorer prognosis than those with recurrent disease (occurrence >6 months after CRT).84–86 APR is recommended for persistent or recurrent disease for salvage therapy, with achievable 5-year local-regional control in up to 30% to 77%87–90 and overall survival rates reported in 24% to 69%.85–91 Positive margins, either microscopic or macroscopic, following resection, male sex, and higher Charlson comorbidity index portend a worse prognosis, whereas younger age (<55 years), T1-2, and N0-1 disease have been associated with higher overall survival following APR.87,92 Major wound complications are common, reported in 36% to 80%,87 although muscle flap use at the time of reconstruction has been associated with significantly lower rates.90
- 2. Systemic chemotherapy should be considered in patients with extrapelvic metastasis or recurrence following surgical salvage. Grade of Recommendation: Strong recommendation based on low-quality evidence, 1C.
Whereas disease localized to the perianal region, anal canal, and regional nodes has a more structured treatment strategy, metastatic disease has no uniform treatment regimen. Platinum-based salvage chemotherapy is commonly offered, although multiple agents have been used.93–95 Limited data with the use of a combination of 5-FU and cisplatin has been reported with response rates of 66%, with median survival of 35 months (actuarial survival at 1 and 5 years of 62% and 32%).96 Biological agents and surgical resection of metastases are largely anecdotal.97 Small case series have demonstrated some success with the use of cetuximab, a monoclonal antibody against epidermal growth factor, alone or in combination with irinotecan for metastatic disease.95,98 Despite these reports, distant metastases are notoriously difficult to treat, and overall median survival is approximately 9 months.99
Management of Inguinal Lymph Node Disease
- 1. Chemoradiation is the treatment of choice for inguinal lymph node disease. Grade of Recommendation: Strong recommendation based on low-quality evidence, 1C.
Similar to management of the primary anal lesion, the mainstay of treatment for concomitant disease of the perirectal or inguinal nodes is chemoradiation. A complete response has been reported in 79% to 92%.51,54,62,72,100 With the identification of any positive inguinal lymph node, bilateral inguinal basins should be incorporated into the radiation fields with the addition of a boost technique. Metachronous lymph nodes are seen in 10% to 20% of patients, normally within 6 months of completing treatment of the primary lesion.101 These metachronous nodes should also be treated with CRT, and typically respond well.102 Elective prophylactic lymphadenectomy is generally not warranted and is associated with high wound complication rates as well as lower-extremity complications.103 Selective inguinal node dissection may be considered for persistent disease following CRT. In small case series, long-term survival has been reported after successful removal of disease.104
Anal Cancer in HIV-Positive Patients
- 1. CD4 counts may be used to predict the outcome and tolerance of CRT in HIV-positive patients: Grade of Recommendation: Weak recommendation based on low-quality evidence, 2C.
The incidence of anal carcinoma is higher in individuals who are HIV-positive105 and continues to rise despite the widespread use of highly active antiretroviral therapy.106,107 Patients with underlying immunosuppression provide additional concerns regarding the ability to tolerate CRT as well as wound healing deficiencies with surgical therapy, and may only tolerate lower doses of chemoradiation.108 Studies comparing immunocompetent and immunosuppressed SCC patients, however, have reported similar overall survival, disease-free survival and colostomy-free survival rates by the use of standard multimodality CRT with MMC and 5-FU when the course of treatment is completed.109,110 There is some controversy regarding CD4 count and correlation with outcome and toxicity, but, in general, this may be used for HIV-positive patients as a general guide along with clinical assessment.109 Patients with CD4 counts >200 cells/mL should typically be treated similarly to non-HIV-infected patients with anal cancer with the use of chemoradiation. CD4 counts <200 cells/mL have been shown to have higher toxicity, and treatment should be individualized.111 Treatment with highly active antiretroviral therapy allows patients to better tolerate CRT and may improve local control,112,113 although higher overall rates of both acute and long-term toxicities have been reported.114,115
Posttreatment Surveillance
- 1. Follow-up examination should typically include anorectal examination including digital rectal examination, anoscopy, and inguinal palpation. Grade of Recommendation: Strong recommendation based on low-quality evidence, 1C.
Anal cancers regress both during and after CRT; therefore, follow-up should generally commence 6 to 12 weeks after the completion of treatment.116,117 Patients should normally be followed up at 3 to 6 months for the first 2 years, 6 to 12 months until 5 years, and annually thereafter, with varying intervals dictated by clinical findings.118 Recurrences are often amenable to further treatment that may result in cure.119 In general, at a minimum anorectal examination should consist of visual inspection, digital rectal examination, and anoscopy, along with inguinal palpation.8 Lesions occurring 3 or more months after the completion of primary CRT are concerning for persistent disease and should be biopsied because digital examination alone is unreliable for confirming residual malignancy.116
- 2. Imaging studies such as EAUS, CT, MRI, and FDG-PET/CT should be considered for posttreatment surveillance to assess for persistent or recurrent disease. Grade of Recommendation: Strong recommendation based on low-quality evidence, 1C.
In addition to physical examination, EAUS has been used to determine response to therapy.120,121 There is some evidence to suggest that EAUS is better than physical examination alone at detecting recurrent disease, with 3-dimensional evaluation more sensitive than traditional 2-dimensional EAUS.122 MRI has not been demonstrated to be a good predictor of clinical response in early (6–8 week) follow-up of CRT,123 whereas longer-term (6–12 months) evaluation demonstrating decreased tumor size and a reduction/stabilization of signal intensity has been associated with improved outcomes in small series.124 There is some evidence that high signal intensity comparable to skeletal muscle may indicate recurrent disease.125 FDG-PET/CT has demonstrated excellent outcomes in the post-CRT setting in differentiating partial response with a complete response126,127 to help guide the need for biopsy for histological confirmation of persistent disease, as well as for the identification of PET-avid recurrences both locally and distant.128 Several laboratory parameters including hemoglobin and biomarkers such as the tumor suppressor genes p53 and p21 have shown some promise in outcome correlation, although, at present, they have a limited role in follow-up.129,130
Anal Margin Squamous-cell Carcinoma
- A disease-specific history and physical examination should be performed, emphasizing risk factors, tumor size, location, and signs of advanced disease. Grade of Recommendation: Strong recommendation based on low-quality evidence, 1C.
Anal margin SCC is essentially a skin malignancy arising between the distal end of the anal canal and a 5-cm margin surrounding the anal verge.17 Patients present with symptoms similar to anal canal tumors, including bleeding, pruritus, or a mass that may resemble skin lesions elsewhere, but often has characteristic rolled everted edges with a central ulceration.4 Staging of anal margin cancers by AJCC criteria follows that of skin cancer elsewhere, based on tumor size and lymph node involvement. Evaluation should consist of a perianal examination, including digital rectal examination, anoscopy, and palpation of the femoral and inguinal lymph node basins.8 T1-3 are staged the same manner as SCC of the anal canal, but T4 signifies invasion of deep extradermal structures such as bone, nerve, striated muscle, or cartilage. N0 and N1 refer to no regional or regional lymph node spread.17 In general, CT of the chest, abdomen, and pelvis should be done to assess for distant metastasis.8
Treatment of anal margin SCC varies depending on size and depth of invasion. In general, T1 and early T2 lesions can be adequately treated with wide local excision (WLE) with a 1-cm margin, although close proximity to the anal canal may make this difficult.4,131,132 Definitive treatment by WLE alone for these early lesions has been associated with 5-year survival rates up to an 88%.133 Primary radiation, combined with chemotherapy, is also an option, albeit less effective than appropriate excision. Small series with radiation alone have demonstrated failure rates of up to 36%.134 Larger cancers usually should be treated with upfront radiation to the inguinal nodal basins along with radiation or excision of the primary tumor. For T3 and T4 lesions, radiation to both inguinal regions and the pelvis along with chemotherapy with the use of 5-FU and MMC or cisplatin should normally be added. APR may be required for larger and deeper, less favorable lesions (T2-4 or N1), those involving the sphincter muscles, patients with incontinence, or patients with multiple recurrences after local excision.132,133 Salvage therapy for treatment failure after local excision can include repeat local excision, APR, and/or radiation therapy with or without chemotherapy. Accounting for size and location, tumors of the anal margin, especially larger ones, are associated with lower rates of overall and colostomy-free survival in comparison with anal canal lesions.31
Low-grade And High-grade Anal Intraepithelial Neoplasia
Despite the predominance of terms for this entity, LGAIN/HGAIN is used because of its similarity to CIN—even sharing pathologic grading criteria for classification.135 It is believed to be a precursor to anal cancer.136 Although a number of risk factors are associated with the development of LGAIN/HGAIN, infection with HPV is the most common, with others including receptive anal intercourse, HIV seropositivity, cigarette smoking, low CD4 counts, and immunosuppression.137–144
Pretreatment Evaluation
- 1. A disease-specific history and physical examination should be performed for LGAIN/HGAIN, emphasizing symptoms, risk factors, and location of disease. Grade of Recommendation: Strong recommendation based on low-quality evidence, 1C.
LGAIN/HGAIN is often found incidentally during surgery for other unrelated problems such as hemorrhoids. However, high-risk populations include men who have sex with men (MSM), HIV-negative women with a history of anal receptive intercourse and/or other HPV-related anogenital malignancies, and immunosuppression such as in patients who have undergone organ transplantation.145 HIV positivity represents not only another high-risk cohort for the existence of LGAIN/HGAIN, approaching 60% in some studies,146 but it also is a risk factor for the progression of the disease to higher grades.147 The pathway of HPV infection leading to CIN, and ultimately, cervical cancer in females parallels that for HPV, LGAIN/HGAIN, and anal squamous-cell cancer.148 This association of HPV with LGAIN/HGAIN has been demonstrated in both males and females.149,150 There are limited data regarding the natural history of untreated LGAIN/HGAIN in the HIV-negative population. Once established in the anal epithelium, it seems that LGAIN/HGAIN rarely regresses, even in HIV-negative individuals.137 The natural history in HIV-positive patients is more ominous. Progression to higher grades (LGAIN to HGAIN) occurs in more than 50% of HIV-positive homosexual males within 2 years.147,151– 153 The risk for progression to invasive cancer ranges from 10% to 50% among HIV-positive patients.152–154
- 2. Anal Papanicolaou smear cytological examination may be useful in the detection and follow-up of LGAIN/HGAIN. Grade of Recommendation: Strong recommendation based on low-quality evidence, 1C.
Screening procedures for LGAIN/HGAIN include anal cytology, colposcopy, biopsy, and high-resolution anoscopy (HRA). Based on numerous similarities between LGAIN/HGAIN and CIN, anal Papanicolaou (Pap) smear cytology consists of using anal swabs to sample cells from the canal and has been instituted for both screening high-risk individuals and as surveillance after treatment for LGAIN/HGAIN. The sensitivity of anal Pap smear evaluation compared with HRA-directed biopsies ranges from 69% to 93% and specificity ranges from 32% to 59%.155–157 Unfortunately, anal cytology in high-risk cohorts such as MSM has false-negative cytology in up to 23% of HIV-negative and 45% for HIV-positive patients.146 Although some economic modeling studies have suggested that frequent anal cytology may be a cost-effective method to prevent anal cancer,158,159 there have not been any randomized or cohort studies to demonstrate improved survival or outcomes. HRA typically involves the application of 3% acetic acid and Lugol iodine solution to the anal canal with evaluation under high-resolution microscopy to help differentiate normal from abnormal tissue. Directed biopsies are performed for any suspicious areas145,160 and for identification of areas that need further treatment.
Treatment
- 1. Observation alone with close clinical follow-up may be considered in select cases for the management of LGAIN/HGAIN. Grade of Recommendation: Weak recommendation based on low-quality evidence, 2C.
There has been considerable debate in the past regarding the optimal treatment strategy for LGAIN/HGAIN. Because of the increased risk of recurrence following treatment, especially in high-risk cohorts such as HIV-positive patients, some have advocated the strategy of expectant management with surveillance every 4 to 6 months.154 Supporters of this “watch and wait” strategy cite overall low rates of disease progression and malignant potential (especially for LGAIN), and the increased morbidity associated with excision and repeated focal destruction. On the other hand, a comprehensive approach with cytology, HRA, targeted biopsies, and directed therapy has reported clearance of HGAIN in up to 80%, with progression to higher-grade disease and invasive cancer in less than 5%.161,162 Therefore, expectant management with close follow-up may be considered in select cases depending on risk factors, comorbidities, and available resources. However, because of the high prevalence of concomitant CIN, a referral to gynecology is recommended to complete the evaluation.163
- 2. Topical 5% imiquimod cream with close long-term follow-up is an appropriate therapy for LGAIN/HGAIN of the anal margin. Grade of Recommendation: Strong recommendation based on low-quality evidence, 1C.
Imiquimod is an immune response modifier with both anti-HPV and antitumor effects. Use of topical 5% imiquimod cream has support from cohort and case series. Pooled analysis has demonstrated a complete response in 48% of patients, with additional partial response in 34% at a mean follow-up of 11 to 39 months.164 Individual series vary, with some series reporting a complete clinical response in 77% to 86%,165,166 despite most of the data being in the high-risk cohort of HIV-positive MSM. Side effects include irritation, burning, and erosions, which may adversely affect patient compliance.167,168 Yet, overall withdrawal rates remain <5%,169 and treatment can often be successfully reinitiated after a break in therapy. Overall recurrence remains a problem, as well as new LGAIN/HGAIN lesions from different HPV serotypes in untreated areas. Therefore, long-term follow-up is essential.170
- 3. Topical 5% 5-FU cream with close long-term follow-up is an appropriate therapy for LGAIN/HGAIN. Grade of Recommendation: Strong recommendation based on low-quality evidence, 1C.
Topical 5-FU was originally used for extramammary Paget disease and has been described for use in LGAIN/HGAIN for 25 years.171–173 Treatment periods typically range from 9 to 16 weeks, although recurrence may be minimized with protracted application.172,174 Initial clinical response rates have been reported in up to 90%, although recurrence may occur in up to 50%.174 Risk factors for recurrence include poorly defined areas of involvement, follicular involvement, poor immune response, dense scar tissue, and recurrent or persistent HPV infection and poor compliance with therapy.175 Local side effects are very common, occurring in up to 85%, and include skin irritation and hypopigmentation, yet rarely result in discontinuation of therapy.174
- 4. Photodynamic therapy with close long-term follow-up may be appropriate therapy for select patients with LGAIN/HGAIN. Grade of Recommendation: Weak recommendation based on low-quality evidence, 2C.
Similar to use in other types of skin cancers, photodynamic therapy has been described in patients with LGAIN/HGAIN since 1992.176 In this process, photosensitizing agents such as 5-aminolevulinic acid creams are applied to the affected area followed by treatment with a specific nanometer wavelength laser. Studies have thus far been limited to case reports and small series, and its ultimate role for patients with LGAIN/HGAIN remains to be determined.177–181
- 5. Targeted destruction and close clinical long-term follow-up is appropriate therapy for LGAIN/HGAIN. Grade of Recommendation: Strong recommendation based on low-quality evidence, 1C.
A variety of approaches to destruction of LGAIN/HGAIN have been described with the goal of preventing disease progression, including WLE and targeted therapy with the use of HRA. Wide local excision is guided by frozen sections of the affected areas, although total excision of all disease is often difficult. One-centimeter margins are used, with large skin and mucosal defects closed with the aid of local flaps. Although anal mapping was once routine and is still used, it is generally not required.182,183 WLE is associated with recurrence rates of 13% to 63%,183–185 and high rates of local wound complications such as stenosis and incontinence.184 Targeted destruction guided by HRA is effective to identify, biopsy, and destroy LGAIN/HGAIN without the morbidity associated with WLE. However, there remains an overall high risk of persistent or recurrent disease, especially among HIV-positive patients, reported in up to 20% to 80%.151,160,186 Surgical complications such as incontinence and stenosis are generally not reported.160 Infrared coagulation has also been used as an effective ablative device and may be associated with less pain in comparison with other tissue destruction techniques. Although individual lesions are successfully destroyed in up to 72% to 81% following initial treatment,187 persistent local disease has been reported in 65% of patients, with similar high recurrence rates, especially in HIV-positive patients.188 Effectiveness in preventing progression to invasive cancer has been demonstrated with either WLE or targeted destruction.188,189
- 6. Patients with LGAIN/HGAIN should be offered close long-term clinical follow-up. Grade of Recommendation: Strong recommendation based on low-quality evidence, 1C.
Patients with LGAIN/HGAIN should typically be monitored for the development of recurrence, persistence, or progression to anal cancer. Surveillance examinations may be performed at 3- to 6-month intervals as long as dysplasia is present.150,154,189 This approach allows for the treatment of recurrent or persistent dysplasia or the detection of invasive anal SCC. Follow-up generally includes digital rectal examination, anoscopic examination, with or without the aid of magnification or the application of acetic acid and Lugol solution, and can be performed in an office setting.190 Anorectal cytology and/or biopsy may also be included, as indicated.161 The importance of close follow-up should be particularly emphasized among high-risk cohorts such as HIV-positive patients, patients wit a history of other HPV-related genital malignancies, recipients of solid organ transplants, or MSM who have been shown to have higher risk of persistence or recurrence of high-grade dysplasia (up to 80%) regardless of treatment modality.160,191
- 7. Vaccination against HPV 16/18 may be considered in high-risk patients such as HIV-positive patients and MSM. Grade of Recommendation: Weak recommendation based on low-quality evidence, 2C.
Approximately 80% of anal SCC is believed to be secondary to HPV serotypes 16 and 18.192 Targeted vaccination against these serotypes, especially in high-risk cohorts, may be able to prevent the development of malignancy.6 Several early reports have documented the safety and efficacy of available vaccines, even in HIV-positive patients.193,194 Similar to studies of HPV in adolescent girls,195 models utilizing vaccination in MSM beginning at age 12 without previous HPV exposure has demonstrated the cost-effectiveness of this approach to prevent infection with HPV, genital warts, and ultimately anal SCC.195 At present, the vaccine make-up, timing of administration, and overall indications continue to be a source of investigation and discussion. Although highly controversial, its ultimate role remains to be determined.
Appendix A: Contributing Members Of The Ascrs Standards Committee
Committee Members: W. Donald Buie, M.D., Chair, Janice Rafferty, M.D., Co-chair, Jose Guillem, M.D., Council Representative, Robin Boushey, M.D., George Chang, M.D., Daniel Feingold, M.D., Philip Fleshner, M.D., Jill Genua, M.D., Kerry Hammond, M.D., William Harb, M.D., Samantha Hendren, M.D., Daniel Herzig, M.D., Andreas Kaiser, M.D., David Larson, M.D., Sang Lee, M.D., James McCormick, D.O., Genevieve Melton-Meaux, M.D., Steven Mills, M.D., John Monson, M.D., Harvey Moore III , M.D., W. Brian Perry, M.D., P. Terry Phang, M.D., David Rivadeneira, M.D., Howard Ross, M.D., Scott Steele, M.D., Scott Strong, M.D., Charles Ternent, M.D., Madhulika Varma, M.D., Martin Weiser, M.D., Kirsten Wilkins, M.D.
References